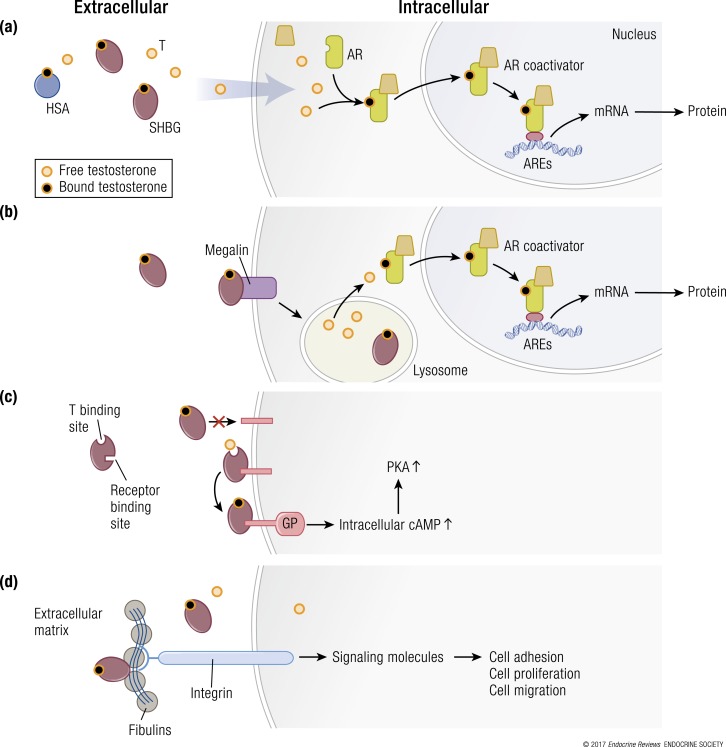
Figure 4.
Multiple hypothetical mechanisms for the cellular uptake of testosterone and downstream signaling. (a) The model depicting the “free” hormone hypothesis. In this model, testosterone (T) that is not bound to SHBG or HSA or other binding proteins diffuses across the plasma membrane and binds to the androgen receptor (AR). The liganded AR recruits coregulators and chaperone proteins, translocates to the nucleus, and binds to androgen response elements (AREs) on androgen-responsive target genes, which activates the transcription of target genes. (b) The megalin-dependent mode of testosterone entry. According to this model, SHBG-bound testosterone is internalized into the cell through an endocytic process mediated by the membrane protein megalin. Once internalized, SHBG-bound testosterone is released at the low pH within the lysosome. (c) The SHBG receptor-testosterone system. The SHBG dimer has multiple binding sites—two sites (simplified as one in this model) bind testosterone, and one site binds to a membrane receptor. It may be that only unbound SHBG is able to bind to the receptor, then the SHBG-receptor-testosterone complex is coupled to the activation of a G protein (GP), the accumulation of intracellular cyclic adenosine monophosphate (cAMP), and activation of protein kinase A (PKA). PKA may modulate AR function by activating AR through phosphorylation (not depicted) (92). (d) Steroid ligand−dependent interactions between SHBG and at least two matrix-associated proteins in the fibulin family (fibulin-1D and fibulin-2) contribute to the extravascular sequestration of SHBG in some tissues, such as the breast, prostate, and endometrial stroma. According to this model, ligand-dependent interactions between SHBG and fibulins modulate their binding to various signaling molecules, such as integrins, to modify signaling pathways that regulate cell adhesion, proliferation, and migration. mRNA, messenger RNA.