Abstract
Introduction
Urinary tract infection (UTI) is a serious health problem affecting millions of people every year. Inappropriate antibiotic prescriptions put patients at risk and lead to bacterial resistance and elevated costs.
Aims
Study aims were to assess the prevalence and antibiotic-treatment patterns of community acquired UTIs, prevalence and types of antibiotic-prescribing errors, and the cost of inappropriate antibiotic use.
Methods
This was a retrospective cross-sectional study conducted over a 3-month period in an emergency department in Saudi Arabia.
Results
During the study period, 1,449 patients were diagnosed with UTIs, including pediatric (18.6%), adult (59.2%), and elderly (22.2%) patients. The overall prevalence of UTIs was 9.9% of total visits. Broad-spectrum antibiotics were prescribed for 85% of patients. Three main antibiotics were prescribed: cephalosporin (39%), penicillin (26%), and fluoroquinolone (22%). The overall prevalence of inappropriate antibiotic prescription with at least one type of error was 46.2% (pediatrics 51%, adults 46%, elderly 47%). Errors were dose (37%), duration (11%), frequency (6%), and antibiotic selection (2.4%). Dose error was significantly greater in pediatric patients (P=0.001). Duration error was higher among adults and the elderly (P=0.014). Significantly more inappropriate cephalosporin prescriptions were seen in adults (P=0.001), while penicillin had significantly higher errors in pediatric patients. Positive urine culture was seen in 34.9% of patients, and the most common microorganism was Escherichia coli (51%). The mean cost of care for one episode of UTI was US$134.56±$31.34 (95% CI $132.94–$136.17). Treatment of UTI was more costly in women (63.9% of total cost), adults (59.2%), and those using broad-spectrum antibiotics (86.5%). There were statistically significant associations among sex, age, spectrum of antibiotic, category of antibiotic, and inappropriate cost.
Conclusion
The results revealed a significant level of inappropriate use of antibiotics in the treatment of UTIs in the emergency department.
Keywords: prevalence, urinary tract infection, antibiotic, cost, inappropriate antibiotic, resistance, antibiotics resistance
Introduction
Urinary tract infections (UTIs) are among the most common infections in the world, affecting all ages – pediatric, adult, and elderly patients – all subpopulations, and both sexes, with highest prevalence in females.1–4 UTIs are a common health problem in primary care, general practice, and emergency departments (EDs). UTI is one of the top 15 diagnoses given annually in the ED and a frequent cause of admission to hospitals. Hospitalization is more common in women and younger children.5–7 The condition is generally associated with minimal morbidity, except among specific subpopulations, such as pediatric patients. Early diagnosis and treatment are essential to reduce acute morbidity and avoid the long-term complications associated with UTIs.2,8
UTIs are a serious health problem affecting millions of people every year, and treatment involves considerable cost, both directly and indirectly. In the US, there werê10.5 million ambulatory visits for UTIs in 2007, accounting for 0.9% of all ambulatory visits, and 21.3% of these visits were to hospital EDs.9 The annual cost of treatment was ~$1.6 billion in 1997,10 and the estimated annual direct treatment cost was $659 million in the US in 1995.8
UTI is one of the most common infections for which antibiotics are prescribed.11–13 The massive and inappropriate use of antibiotics is one of the most important causes of the development of antimicrobial resistance.7,13 The World Health Organization has estimated that 80% of antibiotics are used in the community, and that about 20%–50% of these antibiotics are used inappropriately.14 Antimicrobial resistance is a serious threat to public health throughout the world. It significantly impacts patient treatment and outcomes, increasing health care costs, morbidity, and mortality.13,15,16
Appropriate antibiotic use is a key strategy to control antibacterial resistance. The economic impact of inappropriate treatment extends beyond the costs attributable to morbidity and mortality. Antibiotic therapy is the core treatment for UTIs, so the selected antibiotic should be an efficacious, safe, and cost-effective antimicrobial agent. In Saudi Arabia, one study estimated the prevalence of community-acquired urinary tract infections (CA-UTIs) as 25% of all infections seen in the ED.17
There have been few studies on the cost of UTIs in pediatric and adult patients in other countries, and at the time of this study, there had been no reports published on the costs of management or the appropriateness or otherwise of the treatment of CA-UTIs in any age-group in Saudi Arabia. The aims of this study were thus to determine the prevalence of CA-UTIs among ED visits in Saudi Arabia, assess the pattern of antibiotic treatment of CA-UTIs, determine the prevalence and types of antibiotic-prescribing errors, and assess the cost of inappropriate antibiotic use in the treatment of CA-UTIs.
Methods
Study setting
This study was conducted in the ED of King Abdulaziz Medical City (KAMC). KAMC is a 1,505-bed university-affiliated tertiary care center in Riyadh, Saudi Arabia, and is accredited by Joint Commission International. The ED at KAMC has 132 beds allocated to adult and pediatric wards at various care levels (observation, urgent, emergency, critical). A team of more than 100 emergency-specialized consultants, associate consultants, assistant consultants, staff physicians, and residents provide services to patients.17
Study design
A cross-sectional study was conducted by reviewing the charts of patients in the ED complaining of CA-UTIs over a period of 3 months.
Study population
All patients admitted to the ED complaining of CA-UTIs during the first quarter of the year were enrolled in the study. Patients who fit the study criteria were those aged >6 months and diagnosed with UTI. The UTI was considered community acquired if the patient had not been hospitalized or undergone an invasive urinary tract procedure in hospital during the 2 weeks prior to the appearance of UTI symptoms. The usual criteria that apply to initial UTI diagnosis are history, clinical findings, and a dipstick test. Patients in the study population were classified as pediatric, adult, or elderly. Patients aged <15 years were classed as pediatric, those aged 15–64 years were classed as adults, while those aged ≥65 years or more were classed as elders or older adults. This operational definition was adopted because it had been used in similar studies.7,17 Antibiotic prescriptions that were incomplete and infants weighing <5 kg were excluded from the study. Patients with acute complicated UTI (eg, acute pyelonephritis and UTI with sepsis or bacteremia), catheter-associated UTI, and comorbidities (such as liver disease, renal insufficiency, malignant tumor, and AIDS) were also excluded.
Data collection
Patient characteristics
Demographic data, number of visits to the ED for each patient within 3 months, and recurrence of UTI were assessed. Recurrence was defined as two or more episodes of UTI during the study period.
Antibiotic-prescription characteristics
The name of the antibiotic, the category of antibiotic it belonged to, ie, penicillin, cephalosporin, macrolide, fluoroquinolones, sulfonamides, and miscellaneous, which included clindamycin, nitrofurantoin, and doxycycline, and dose, frequency, duration of antibiotic therapy, and cost were reviewed.
Microbiology characteristics
Urine cultures collected during ED visits in the study period, culture results (positive or negative), and names of microorganisms were reviewed. Positive urine culture was defined as bacterial growth of 105 CFU/mL urine of a single bacterium from a properly collected midstream “clean catch” urine sample. Bacterial isolates were tested for their antimicrobial susceptibility and interpreted by a modified Vitek 2 method, according to the guidelines of the Clinical and Laboratory Standards Institute. Sensitivity and resistance of Escherichia coli isolates to six antimicrobial agents – ampicillin, amoxicillin–clavulanic acid (Augmentin), co-trimoxazole (trimethoprim–sulfamethoxazole), ciprofloxacin, nitrofurantoin, and cefazolin – were determined. Multiresistance was defined as acquired resistance to at least three classes of antibiotic.
Outcome characteristics
There were two study outcomes: inappropriateness of antibiotic treatment and cost of treatment.
Inappropriateness of antibiotic treatment
Four main ways were identified in which antibiotic treatment could be inappropriate: errors in selection, dosage, frequency, and duration. Inappropriateness of antibiotic treatment was thus defined as selection of an antibiotic that was neither the drug of choice nor the alternative drug indicated for the disease, or prescription of an inappropriate dose, frequency between doses, or duration of treatment. Inappropriate dose was defined as either more or less than the recommended daily amount of the antibiotic. Inappropriate frequency was defined as more or less than the recommended daily frequency. Inappropriate duration was defined as shorter or longer than the recommended duration. For inappropriate dose and inappropriate duration, variability of ±5% was allowed between the prescribed and recommended dose and duration, with variation beyond this margin identified as inappropriate. Each antibiotic prescription was evaluated for appropriateness according to the guidelines stated in American Hospital Formulary Service Drug Information from the American Society of Health-System Pharmacists and the Drug Information Handbook: A Comprehensive Resource for All Clinicians and Health Care Professionals.18,19
Estimates of treatment cost
Only costs charged in the ED were taken into account for patients admitted and discharged from the ED. The total direct cost to the hospital of treatment of UTIs was analyzed. Indirect costs, such as those associated with sickness, were not included. Treatment costs did not include the price of medical equipment used. US dollars were used to calculate the costs recorded in this study. Direct treatment costs included medical costs relating to physicians’ fees, diagnostic tests (only tests performed for UTIs were considered), and prescription drugs (only antibiotics; over-the-counter drugs were not taken into account). The direct costs of clinical UTIs were estimated using data from the business center. Prescriptions are generally free for eligible patients.
Data management and analysis
SPSS statistical software (version 22; IBM, Armonk, NY, USA) was used for data entry and analysis. Bivariate analysis using Pearson’s χ2 test was used for categorical data, such as age-group, sex, and antibiotic category. Prevalence of inappropriate antibiotic prescriptions was determined as the number of physician orders with one or more types of error divided by the total number of prescriptions and multiplied by 100. The prevalence of errors (selection, dose, frequency, and duration) was first calculated as mutually exclusive prevalence by dividing the number of errors over the number of antibiotic prescriptions multiplied by 100.
For cost, data were summarized in the form of mean ± standard deviation (SD) or median (range) for continuous variables, and numbers and percentages for categorical variables wherever appropriate, with χ2 used for categorical variables. For all statistical tests, P<0.05 was considered statistically significant.
Ethics statement
This study was approved by the research committee at King Abdullah International Medical Research Center (KAIMRC), King Saud Bin-Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia (RR08/005). Patient informed consent to review their medical files was not required and waived by the research committee at KAIMRC, since this was a retrospective study and there was no communication with patients. Patient privacy and confidentiality of data were secured by the investigator.
Results
General characteristics
During the study period, a total of 1,449 patients were diagnosed with UTIs. These included pediatric (18.6%), adult (59.2%), and elderly (22.2%) patients. Female patients made up the majority, 64.04%, of all patients visiting the ED with a UTI. The male:female ratio was 1:1.8, and 70.29% of all adults, 67.84% of pediatric patients, and 43.48% of elderly patients were female. The prevalence of recurrent episodes of infection was 18% in the study population as a whole. It was highest in female patients, particularly pediatric and adult female patients, but less so in elderly female patients (80%, 73%, and 42.9%, respectively). This difference was statistically significant for females of all age-groups (Table 1).
Table 1.
Bivariate analysis of patient characteristics and antibiotic prescriptions by age-group
| Sample, n (%) (total 1,449) | Pediatric, n (%) (total 269 [18.6]) | Adult, n (%) (total 858 [59.2]) | Elderly, n (%), (total 322 [22.2]) | χ2, P-value | |
|---|---|---|---|---|---|
|
| |||||
| Sex | 80.866, <0.05 | ||||
| Male | 521 (36) | 96 (35.7) | 243 (28.3) | 182 (56.5) | |
| Female | 928 (64) | 173 (64.3) | 615 (71.7) | 140 (43.5) | |
| UTI frequency within 3 months | 60.368, <0.001 | ||||
| One | 1,187 (81.9) | 238 (88.5) | 732 (85.3) | 217 (67.4) | |
| Recurrent | 262 (18.1) | 31 (11.5) | 126 (14.7) | 105 (32.6) | |
| Urine-culture request at ED | 2.049, 0.359 | ||||
| Yes | 565 (39) | 115 (42.8) | 325 (37.9) | 125 (38.8) | |
| No | 884 (61) | 154 (57.2) | 533 (62.1) | 197 (61.2) | |
| Result of culture | 9.903, <0.05 | ||||
| Positive | 197 (34.9) | 30 (26.1) | 128 (39.4) | 39 (31.2) | |
| Negative | 368 (65.1) | 85 (73.9) | 197 (60.6) | 86 (68.8) | |
| Antibiotics within 3 months, n | 71.917, <0.001 | ||||
| 1 | 1,187 (81.9) | 238 (88.5) | 732 (85.3) | 217 (67.4) | |
| 2 | 203 (14) | 28 (10.4) | 103 (10.4) | 72 (22.4) | |
| ≥3 | 59 (4.1) | 3 (1.1) | 23 (2.7) | 33 (10.2) | |
| Antibiotic group | 185.472, <0.001 | ||||
| Cephalosporin | 567 (39.1) | 117 (43.5) | 322 (37.5) | 128 (39.8) | |
| Macrolide | 39 (2.7) | 8 (3) | 19 (48.7) | 12 (3.7) | |
| Penicillin | 379 (26.3) | 119 (44.2) | 226 (26.3) | 34 (10.6) | |
| Tetracycline | 3 (0.2) | 0 | 2 (0.2) | 1 (0.3) | |
| Miscellaneous | 70 (4.8) | 3 (1.1) | 61 (7.1) | 6 (1.9) | |
| Fluoroquinolone | 327 (22.6) | 7 (2.6) | 190 (22.1) | 130 (40.4) | |
| Sulfonamide | 64 (4.4) | 15 (5.6) | 38 (4.4) | 11 (3.4) | |
| Antibiotic spectrum | 46.101, <0.001 | ||||
| Narrow | 206 (14.2) | 66 (24.5) | 124 (14.5) | 16 (5) | |
| Broad | 1,243 (85.8) | 203 (75.5) | 734 (85.5) | 306 (95) | |
Abbreviations: ED, emergency department; UTI, urinary tract infection.
Prevalence of UTIs
The overall prevalence of UTIs was 9.9% of total visits to the ED. In adults and elders, UTIs accounted for ~14.6% of total ED visits. However, UTIs in pediatric patients accounted for ~4% of all pediatric ED visits.
Urine culture and sensitivity
Urine culture was requested for 39% of patients. Of the total 565 urine cultures, 197 were positive (34.9% of all requested cultures) and 368 negative (65.1%). There were significant differences in numbers of positive and negative culture results between age-groups (P<0.05; Table 1). E. coli was the principal microorganism responsible for UTIs. It was the most common urinary pathogen seen in positive cultures (51%), and resistance to commonly used antibiotics was seen in 68.32% of these cases. E. coli was highly sensitive to nitrofurantoin (92%), followed by ciprofloxacin (81.8%), amoxicillin–clavulanic acid (81.1%), and cefazolin (77.5%). There was a decreasing level of sensitivity of E. coli to co-trimoxazole (55.6%), followed by ampicillin (33.1%).
Antibiotic treatment
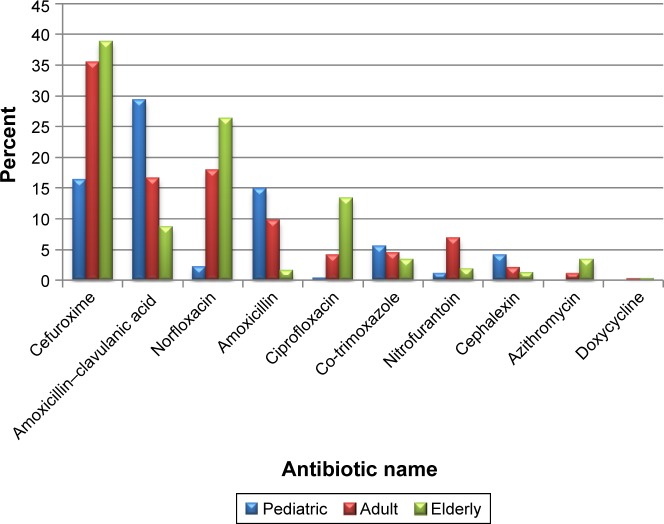
Broad-spectrum antibiotics were prescribed for 85.8% of cases. Seven classes of antibiotic were used in the treatment of UTIs, but three main antibiotic classes were commonly prescribed: cephalosporin (39%), penicillin (26%), and fluoroquinolones (22%), with significant differences among the three age-groups (Table 1). Cefuroxime, amoxicillin–clavulanic acid, norfloxacin, and amoxicillin were the antibiotics most commonly used overall for UTIs (Figure 1).
Figure 1.
Distribution of antibiotics prescribed by age-group.
The antibiotics most often prescribed in pediatric patients were amoxicillin–clavulanic acid (29.37%), followed by cefprozil (23%), cefuroxime (16.36%), amoxicillin (14.9%), co-trimoxazole (5.8%), cephalexin (4.1%), and ciprofloxacin (0.37%).
In adults, the most frequently prescribed antibiotics were cefuroxime (35.4%), norfloxacin (17.9%), and amoxicillin– clavulanic acid (16.6%), followed by amoxicillin (9.8%), nitrofurantoin (6.9%), co-trimoxazole (4.4%), and ciprofloxacin (4.1%), and the least commonly prescribed in adults were cephalexin (2.1%), and azithromycin (1%).
In the elderly, the most frequently prescribed antibiotics were cefuroxime (38.8%), norfloxacin (26.4%), and ciprofloxacin (13.4%), followed by amoxicillin–clavulanic acid (8.7%), azithromycin (3.4%), and co-trimoxazole (3.4%), and the least commonly prescribed were nitrofurantoin (1.9%), amoxicillin (1.6%), and cephalexin (1.2%). There were statistically significant differences among age-groups in the types of antibiotics used (Table 1). The majority of patients (81.9%) received one course of antibiotics for the treatment of UTI during the study period, but at least 4% of patients received three or more courses of antibiotics for recurrent UTIs during the same period (Table 1).
Prevalence of inappropriate treatment
The prevalence of inappropriate antibiotic prescriptions with at least one or more types of error was 47.3% (685 cases), and this figure was higher in pediatric patients (51.3%) than in adults (46%) and the elderly (47.2%; Table 2). The mutually exclusive prevalence of different types of error showed that dose errors were most frequent (37.5%), followed by duration errors (11%), frequency errors (6.1%), and finally inappropriate selection of antibiotic class (2.4%).
Table 2.
Prevalence of inappropriate antibiotic prescriptions and types of error by age-group
| Characteristics | Pediatric, n (%) (total 269 [18.6]) | Adult, n (%) (total 858 [59.2]) | Elderly, n (%) (total 322 [22.2]) | Total, n (%) | χ2, P-value |
|---|---|---|---|---|---|
| Inappropriate antibiotic | 138 (51.3) | 395 (46) | 152 (47.2) | 685 (47.3) | 3.277, 0.32 |
| Type of error | |||||
| Dose error | 119 (44.2) | 305 (35.5) | 120 (37.3) | 544 (37.5) | 6.609, <0.05 |
| High dose | 77 (28.6) | 296 (34.5) | 116 (36) | 489 (33.7) | |
| Low dose | 11 (4.1) | 9 (1) | 4 (1.2) | 24 (1.7) | |
| Others | 31 (11.5) | 31 (11.5) | |||
| Frequency error | 16 (5.9) | 58 (6.8) | 15 (4.7) | 89 (6.1) | 1.815, 0.403 |
| High | 4 (1.5) | 16 (1.9) | 7 (2.2) | 27 (1.9) | |
| Low | 12 (4.5) | 42 (4.9) | 8 (2.5) | 62 (4.3) | |
| Duration error | 17 (6.3) | 98 (11.4) | 44 (13.7) | 159 (11) | 8.527, <0.05 |
| Long | 4 (1.5) | 24 (2.8) | 9 (2.8) | 37 (2.6) | |
| Short | 13 (4.8) | 74 (8.6) | 35 (10.9) | 122 (8.4) | |
| Selection error | 9 (3.3) | 19 (2.2) | 7 (2.2) | 35 (2.4) | 1.1214, 0.545 |
| Cost of inappropriate antibiotic (US$), mean ± SD | 21.89±12.63 | 25.12±13.76 | 25.88±12.01 | 24.73±13.25 | |
| 95% CI | 19.77–24.02 | 23.96–27.81 | 23.77–26.46 | 23.73–25.72 | P<0.05 |
| Cost of inappropriate antibiotics, % | 47.4 | 47.1 | 48.2 | 47.5 | |
Pediatric patients were exposed to a significantly higher prevalence of inappropriate antibiotic dose (P<0.05), whereas in adults and elderly patients there was a significantly higher prevalence of inappropriate duration of antibiotic treatment (P<0.05). There was no significant difference among age-groups in regard to prevalence of inappropriate frequency (P=0.403; Table 2).
Predictors of antibiotic-prescription errors
Initial bivariate analysis of all age-groups showed that 90.7% of cephalosporin prescriptions for adults contained errors, significantly higher than for cephalosporin prescribed for elderly (83.6%) and pediatric (66.7%) patients (P<0.001). Penicillin prescriptions for pediatric patients had significantly more errors (33.6%) than those prescribed for other age-groups (P<0.001). Narrow-spectrum antibiotic prescriptions in pediatric patients had significantly more errors (45.5%) than those in elderly (43.8%) and adult (23.8%) patients (P<0.05; Table 3).
Table 3.
Bivariate analysis of inappropriate antibiotic prescriptions by age-group
| Characteristics | Sample, n (%) (total 1,449) | Pediatric, n (%) (total 269 [18.6]) | Adult, n (%) (total 858 [59.2]) | Elderly, n (%) (total 322 [22.2]) | Total, n (%) | χ2, P-value |
|---|---|---|---|---|---|---|
| Inappropriate antibiotic | 138 (51.3%) | 395 (46%) | 152 (47.2%) | 685 (47.3%) | 3.277, 0.32 | |
| Sex | ||||||
| Male | 521 (36) | 50 (52.1) | 99 (40.7) | 87 (47.8) | 236 (45.3) | 4.281, 0.118 |
| Female | 928 (64) | 88 (50.9) | 296 (48.1) | 65 (46.4) | 449 (48.4) | 0.657, 0.720 |
| Request for urine culture at ED | ||||||
| Yes | 565 (39) | 68 (59.1) | 145 (44.6) | 57 (45.6) | 270 (47.8) | 7.480, <0.05 |
| No | 884 (61) | 70 (45.5) | 250 (46.9) | 95 (48.2) | 415 (46.9) | 0.267, 0.875 |
| Result of culture | ||||||
| Positive | 197 (13.6) | 19 (63.3) | 64 (50) | 23 (59) | 106 (53.8) | 2.261, 0.323 |
| Negative | 1,252 (86.4) | 119 (48.8) | 331 (45.3) | 129 (45.6) | 579 (46.2) | 1.498, 0.473 |
| Antibiotics within 3 months, n | ||||||
| 1 | 1,187 (81.9) | 121 (50.8) | 338 (46.2) | 108 (49.8) | 567 (47.8) | 1.993, 0.369 |
| 2 | 203 (14.2) | 17 (60.7) | 46 (44.7) | 31 (43.1) | 94 (46.3) | 2.756, 0.252 |
| ≥3 | 59 (4.1) | 0 | 11 (47.8) | 13 (39.4) | 24 (40.7) | 2.567, 0.277 |
| Frequency of UTI within 3 months | ||||||
| One | 1,187 (81.9) | 121 (50.8) | 338 (46.2) | 108 (49.8) | 567 (47.8) | 1.993, 0.369 |
| Recurrent | 262 (18.1) | 17 (54.8) | 57 (45.2) | 44 (41.9) | 118 (45) | 1.621, 0.445 |
| Antibiotic group | ||||||
| Cephalosporin | 567 (39.1) | 78 (66.7) | 292 (90.7) | 107 (83.6) | 477 (84.1) | 37.104, <0.001 |
| Penicillin | 379 (26.3) | 40 (33.6) | 24 (10.6) | 2 (5.9) | 66 (17.4) | 32.112, <0.001 |
| Fluoroquinolone | 327 (22.6) | 0 | 29 (15.3) | 27 (20.8) | 56 (17.1) | 3.127, 0.209 |
| Macrolide | 39 (2.7) | 6 (75) | 6 (31.6) | 5 (41.7) | 17 (43.6) | 4.343, 0.114 |
| Nitrofurantoin | 70 (4.8) | 3 (100) | 25 (41) | 4 (66.7) | 32 (45.7) | 5.174, 0.075 |
| Sulfonamide | 64 (4.4) | 11 (73.3) | 19 (50) | 7 (63.6) | 37 (57.8) | 2.585, 0.275 |
| Antibiotic spectrum | ||||||
| Narrow | 206 (14.2) | 30 (45.5) | 29 (23.4) | 7 (43.8) | 66 (32) | 10.726, <0.05 |
| Broad | 1,243 (85.8) | 108 (53.2) | 366 (49.9) | 145 (47.4) | 619 (49.8) | 1.654, 0.437 |
Abbreviations: ED, emergency department; UTI, urinary tract infection.
UTI costs
The cost of inappropriate antibiotic use represented 47.5% of the total cost of antibiotic treatment, with a mean cost per UTI episode of $24.73±$13.25 (95% CI $23.73–$25.72) (P<0.05), and a range of $1.17–$87.47 (Table 2). The cost of hospital treatment ranged from $90.19 to $328.65 per patient, estimated total direct cost of UTI treatment for all patients who visited the ED was $194,971, and annual total hospital-management cost was estimated as $838,375. The median cost of hospital treatment was $118.61, and the mean was $134.56±$31.34 (95% CI $132.94–$136.17). The total management cost for UTIs was greater for female patients (63.9% of total cost), adults (59.2%), cephalosporin (40.9%), and broad-spectrum antibiotics (86.5%; Table 4).
Table 4.
Total cost (US$) of treatment for community-acquired urinary tract infection
| Characteristics | n | % | Mean | SD | Minimum | Maximum | Median | Cost | % cost | Std. error | P-value | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age-group | |||||||||||||
| Adult | 858 | 59.2 | 134.52 | 31.20 | 90.19 | 328.65 | 118.61 | 115,414.12 | 59.2 | 1.07 | 0.599 | 132.42 | 136.65 |
| Elderly | 322 | 22.2 | 135.81 | 31.22 | 90.19 | 236.27 | 126.61 | 43,729.50 | 22.4 | 1.74 | 132.54 | 139.33 | |
| Pediatric | 269 | 18.6 | 133.19 | 31.96 | 90.19 | 193.12 | 118.61 | 35,827.55 | 18.4 | 1.95 | 129.45 | 137.22 | |
| Sex | |||||||||||||
| Male | 521 | 36.0 | 135.15 | 31.21 | 90.19 | 236.27 | 126.40 | 70,412.79 | 36.1 | 1.37 | 0.589 | 132.53 | 138.00 |
| Female | 928 | 64.0 | 134.22 | 31.42 | 90.19 | 328.65 | 118.61 | 124,558.38 | 63.9 | 1.03 | 132.06 | 136.25 | |
| Single or recurrent UTI | |||||||||||||
| Single | 1,187 | 81.9 | 134.57 | 31.45 | 90.19 | 328.65 | 118.61 | 159,729.70 | 81.9 | 0.91 | 0.979 | 132.78 | 136.27 |
| Recurrent | 262 | 18.1 | 134.51 | 30.88 | 90.19 | 193.12 | 126.40 | 35,241.47 | 18.1 | 1.91 | 131.19 | 138.22 | |
| Urine culture | |||||||||||||
| Yes | 565 | 39.0 | 166.21 | 20.46 | 90.19 | 328.65 | 169.68 | 93,910.99 | 48.2 | 0.86 | <0.001 | 164.55 | 168.00 |
| No | 884 | 61.0 | 114.32 | 17.09 | 90.19 | 187.73 | 113.68 | 101,060.18 | 51.8 | 0.57 | 113.24 | 115.50 | |
| Result of culture | |||||||||||||
| Negative | 368 | 65.1 | 166.33 | 17.79 | 95.28 | 236.27 | 169.68 | 61,209.56 | 31.4 | 0.93 | <0.001 | 164.51 | 168.15 |
| Positive | 197 | 34.9 | 166.00 | 24.74 | 90.19 | 328.65 | 169.68 | 32,701.43 | 16.8 | 1.76 | 162.52 | 169.47 | |
| Diagnostic test | |||||||||||||
| No | 884 | 61.0 | 112.07 | 13.36 | 90.19 | 175.47 | 112.56 | 99,066.60 | 50.8 | 0.45 | <0.001 | 111.18 | 112.95 |
| Yes | 565 | 39.0 | 169.74 | 14.47 | 145.17 | 328.65 | 174.61 | 95,904.57 | 49.2 | 0.61 | 168.55 | 170.94 | |
| Number of antibiotics | |||||||||||||
| 1 | 1,187 | 81.9 | 134.57 | 31.45 | 90.19 | 328.65 | 118.61 | 159,729.70 | 81.9 | 0.91 | 0.126 | 132.77 | 136.36 |
| 2 | 203 | 14.0 | 136.63 | 30.71 | 90.19 | 193.12 | 131.73 | 27,736.80 | 14.2 | 2.16 | 132.38 | 140.88 | |
| ≥3 | 59 | 4.1 | 127.20 | 30.61 | 90.19 | 187.73 | 118.61 | 7,504.67 | 3.8 | 3.98 | 119.22 | 135.17 | |
| Antibiotic group | |||||||||||||
| Cephalosporin | 567 | 39.1 | 140.59 | 29.95 | 90.19 | 236.27 | 131.73 | 79,715.38 | 40.9 | 1.26 | <0.001 | 138.00 | 142.99 |
| Macrolide | 39 | 2.7 | 129.21 | 31.77 | 91.06 | 182.61 | 109.87 | 5,039.35 | 2.6 | 5.09 | 119.02 | 140.35 | |
| Penicillin | 379 | 26.2 | 132.60 | 31.69 | 90.19 | 328.65 | 118.61 | 50,255.32 | 25.8 | 1.63 | 129.56 | 135.73 | |
| Tetracycline | 3 | 0.2 | 128.76 | 17.59 | 118.61 | 149.07 | 118.61 | 386.29 | 0.2 | 10.15 | 124.70 | 141.46 | |
| Miscellaneous | 70 | 4.8 | 130.42 | 29.80 | 91.01 | 193.12 | 118.61 | 9,129.24 | 4.7 | 3.56 | 123.51 | 138.12 | |
| Fluoroquinolone | 327 | 22.6 | 129.76 | 32.52 | 90.19 | 209.15 | 118.61 | 42,431.70 | 21.8 | 1.80 | 126.19 | 133.28 | |
| Sulfonamide | 64 | 4.4 | 125.22 | 28.87 | 91.06 | 176.57 | 118.61 | 8,013.89 | 4.1 | 3.61 | 118.09 | 132.73 | |
| Antibiotic spectrum | |||||||||||||
| Narrow | 206 | 14.2 | 127.68 | 32.40 | 91.06 | 328.65 | 118.61 | 26,302.26 | 13.5 | 2.26 | <0.05 | 123.25 | 132.38 |
| Broad | 1,243 | 85.8 | 135.70 | 31.03 | 90.19 | 236.27 | 126.40 | 168,668.91 | 86.5 | 0.88 | 133.89 | 137.34 | |
| Total | 1,449 | 100.0 | 134.56 | 31.34 | 90.19 | 328.65 | 118.61 | 194,971.17 | 100.0 | 0.82 | 132.94 | 136.17 | |
Abbreviations: UTI, urinary tract infection; Std. error, standard error.
The cost of antibiotics ranged from $1.17 to $92.27 per patient, total direct cost was $35,629, and the annual total cost of antibiotics was estimated as $153,208 per year. When urine cultures were performed, 34.9% of these gave positive results. The mean cost per patient differed significantly between those with positive and negative urine cultures (P<0.001). The total cost for suspected UTIs with negative urine cultures was 31.4% of total UTI-management costs. The mean cost per UTI episode without diagnostic testing was $112.06±$13.36 (95% CI $111.18–$112.95) compared to a cost with diagnostic testing of $169.74±$14.46 (95% CI $168.54–$170.94, P<0.001).
Comparative analysis showed differences in the total direct cost among various groups. There was a statistically significant difference in the cost of appropriate vs inappropriate antibiotic treatment (P<0.001), and also within the categories of antibiotic spectrum (P<0.001), antibiotic category (P<0.001), antibiotic itself (P<0.001), diagnostic test, and culture results (P<0.001). No statistically significant differences in inappropriate cost were found within the categories of age or sex.
Discussion
This study provides new information in three areas: prevalence of UTIs among ED visits, prevalence of inappropriate prescribing of antibiotics, and cost of treatment, including the cost of inappropriate treatment of UTIs. The prevalence of UTIs varies widely among countries, due to many factors, such as geographical location, age, sex, socioeconomic status, and health status. In this study, UTIs accounted for almost 9.9% of emergency visits during one quarter of the year. In pediatric emergency visits, the prevalence of UTIs was 4%, slightly less than in previous studies conducted in other countries, in which this figure ranged between 5% and 14% of ED visits annually.20,21 In the US, pediatric patients diagnosed with a UTI account for over 1 million office visits and 500,000 ED visits annually, representing around 2%–3% of all pediatric hospitalizations.2,21
In the present study, the prevalence of UTIs among adult and elderly patients was almost 14.6% of emergency visits over one quarter of the year. This was slightly more than the 11% seen in another study conducted in Saudi Arabia.22 In comparison, a study in the US found 5% of elders and 3% of adults visiting the ED with a diagnosis of UTI.4 The prevalence of UTI among adult and the elderly in Spain was 41%.7 In France, the yearly number of emergency visits for UTIs is estimated to be 2.3%.3
In this study, female patients had a relatively high frequency of UTIs compared to male patients, possibly due to anatomical and physiological differences. These results are consistent with data from large epidemiological UTI studies.7,10,23 UTI is one of the most frequent clinical bacterial infections in female patients: approximately one in three women will have at least one symptomatic UTI and require antimicrobial treatment for a UTI before age 24 years, and 50%–60% of women will have a UTI during their lifetime.8,10 According to a study by Foxman et al,10 10.8% of women over 18 years of age have at least one UTI per year. Women represented more than 8.5 million of the population of Saudi Arabia as of January 2016.24 In this study, the estimated number of women who visited primary care for a UTI was estimated as 880,000 in 2016. The yearly estimated number of emergency visits for UTIs is 440,000, half the number for primary care.3
In the present study, the frequency of UTIs in women decreased with age, but increased in elderly men. However, in another study, a similar frequency of UTIs was seen in elderly patients of both sexes.7 The prevalence of UTIs among pediatric male patients decreased rapidly with age in another study.20 In this study, recurrent episodes made up 18.1% of all cases of UTI. The frequency of recurrence of UTI was greater in female than in male patients. Recurrent UTIs have been shown to affect 25%–30% of healthy young women with anatomically and physiologically normal urinary tracts. This may be due to genetic and behavioral factors.25 Recurrent UTIs in pediatric patients represented 11% of all pediatric UTI cases in this study. This was less than in previous studies in Saudi Arabia, where recurrent episodes were 45%–65%.26,27
Negative results were reported in two-thirds of urine cultures, and suspected UTIs with negative urine cultures had a significant influence on cost in this study, accounting for 31% of the total cost. In another study, conducted in general practice, approximately a quarter of suspected UTIs had negative urine cultures.28 The sensitivity and specificity of urine-dipstick tests positive for leukocytes or nitrites in diagnosing UTIs in unpregnant female patients have been reported as 51% and 99%, respectively.29 A positive nitrite test and pyuria are most strongly correlated with UTIs, and increase the probability that a UTI is present more than sevenfold.28 The urine dipstick may be more valuable in ruling out disease, ie, negative leukocytes and nitrites rule out UTIs. When there is doubt as to the cause of the clinical presentation or when the history may indicate a different diagnosis, a urine culture is recommended to confirm the diagnosis of UTI.28,29 Evaluation of clinical signs and symptoms together with the results of pyuria and nitrituria in the reactive strip test may help physicians make relevant decisions before UTI is confirmed by culture results.28
In the present study, E. coli was the most prevalent pathogen contributing to UTI. It was resistant to commonly used antibiotics in the majority of cases. The highest resistance was seen with ampicillin and co-trimoxazole, and least resistance with nitrofurantoin. These findings were similar to those in previous studies.7,22,26,30–34 The prevalence of resistance to co-trimoxazole and ampicillin was increased significantly among microorganisms causing UTIs, and resistance to nitrofurantoin and ciprofloxacin remained infrequent in other studies.23,32,33,35
The etiology of UTIs and antimicrobial-resistance patterns change from country to country and over time, and may increase or decrease. Emergency medicine is not a specialty, and hence doctors attending emergency areas are usually general physicians and residents. Prescribing physicians are thus probably aware that resistance rates of E. coli to co-trimoxazole and ampicillin are significantly higher in complicated CA-UTIs.7
Cephalosporin, penicillin, and fluoroquinolones were the most common antibiotics used to treat UTIs in this study. This is similar to other studies, because of the broad antimicrobial activity, clinical efficacy, and favorable tolerability profiles of these antibiotics.13,23 In this study, cefuroxime and amoxicillin–clavulanic acid were the antibiotics prescribed most often in adults, whereas cefuroxime and fluoroquinolones were most often prescribed in the elderly. There was a low percentage of co-trimoxazole prescriptions in adults and the elderly, as in previous studies.4,7,13 In pediatric patients, amoxicillin–clavulanic acid and amoxicillin were the antibiotics most frequently prescribed, similar to a large-scale survey of treatment for UTIs in children in primary- and secondary-care practices in Europe.36
In some countries, co-trimoxazole, fluoroquinolones, and nitrofurantoin are the most commonly selected antibiotics for UTIs. However, over the course of 10 years of observation, the use of co-trimoxazole declined, while the use of the other two agents rose.23 Patterns of antibiotic prescription for the treatment of UTIs may vary among different countries according to the patient’s age and sex, any underlying diseases, physician preference/familiarity, patient allergies, local resistance patterns, patient population, side effect profile, and cost.4,32,37,38
The ED is one of the service units where antibiotic treatment is commonly prescribed.7,11,12,37 Overuse and inappropriate antimicrobial treatment in the ED is relatively frequent. Using less than the recommended treatment regimen puts patients at risk of ineffective treatment and developing resistant bacteria, and using more than the recommended regimen puts them at risk of side effects.
There is significant variability among countries in prescribing errors for UTIs. The prevalence of error in this study was 47.3%, which was similar to a Turkish study,37 greater than a Spanish study (13%),7 within the range of two French studies (31%–60%),39,40 and less than in a German study (61%).41 The prevalence of errors was found to be greater in patients with cephalosporin prescriptions and broad-spectrum antibiotics, as had been shown in a previous study.17
Inappropriate drug selection, such as the use of macrolides and moxifloxacin for the treatment of UTIs, was seen in 2.7% of cases. Azithromycin is indicated for the treatment of urethritis, and macrolides are not active against UTI pathogens, such as E. coli.37 Moxifloxacin has limited concentration in the urinary tract.42 Neither macrolides nor moxifloxacin are recommended for the treatment of UTIs.
A cost analysis was performed to evaluate the mean quarterly direct treatment cost for CA-UTIs and the costs of inappropriate antibiotic treatment for all age-groups. This was an initial study conducted in Saudi Arabia on the costs of CA-UTIs in all age-groups. The cost of treatment was reimbursed by the Ministry of the National Guard to employees and their dependents. The annual costs, derived from the study results by multiplying the 3-month values by 4.33 (52/12), revealed that the total hospital-management cost for UTIs was estimated as $838,375 per year and total antibiotic cost was estimated as $153,208 per year.
In this study, the mean cost of treatment of CA-UTI was estimated as $134.56 per UTI episode over a quarter of the year, which was different from the cost calculated in previous studies conducted in other countries, such as the UK,1 France,3 Spain,7 the US,5 and Italy.43 The differences in cost between this and other studies may be due to differences in study design: this study was conducted in all age-groups and both sexes, whereas the other studies were conducted only in women. Costs of physician visits and diagnostic tests were greater in Europe and the US than in Saudi Arabia. In addition, populations in Europe and the US are larger than the Saudi population.
Overall, the treatment of CA-UTIs was costly because of high resistance and because patients with CA-UTI may need to have urinary cultures carried out to direct appropriate antimicrobial therapy in regard to reconsultations and antibiotics prescribed. The selection of broad-spectrum agents leads to higher antibiotic costs.
There was a statistically significant difference in the distribution of inappropriate costs between patients who had undergone diagnostic tests and those who had not. Patients who had undergone diagnostic tests were wrongly prescribed expensive antibiotics, leading to higher inappropriate costs than for patients who had not undergone diagnostic tests but who were also wrongly prescribed antibiotics.
Limitations
This study has several limitations. First, it was conducted in only one setting, the ED, which does not represent other health care settings in the KAMC, and thus the findings cannot be generalized. Second, this was a short retrospective study (3 months) that did not include all cases. In addition, some cases may have been lost due to the definition of a positive urine culture as bacterial growth of 105 CFU/mL with a single bacterium. Finally, other limitations may have resulted in over- or underestimation of the costs of UTIs. Large-scale prospective studies are recommended to determine more accurately the prevalence and cost of UTIs at KAMC in Saudi Arabia.
Conclusion
In this study, UTIs were one of the most frequent infections requiring a visit to the ED. The results showed a high level of inappropriate use of antibiotics in the treatment of UTIs in the ED, especially among pediatric and elderly patients. They also showed a significant increase in the prevalence of resistance to several commonly used antimicrobials, such as ampicillin and co-trimoxazole. These findings demonstrate that development of guidelines for the treatment of UTIs should be based on knowledge of the local prevalence of bacterial organisms and their sensitivities, rather than on universal guidelines. More appropriate antibiotic treatment will increase efficacy and cost-effectiveness. It is necessary to improve physicians’ prescribing habits by implementing medical education programs focusing on the rational use of antibiotics. Additional studies should be performed in the area of cost-effective strategies for the treatment of UTIs in Saudi Arabia. Prevalence and antibiotic-susceptibility studies need to be conducted regularly, and this will facilitate early detection of the development of antibiotic resistance and enable guidelines to be developed for appropriate and cost-effective treatment of UTIs.
Acknowledgments
The author would like to acknowledge the King Abdullah International Medical Research Center (KAIMRC) for its support. Special thanks go to the KAIMRC Publication Office, Dr Ahmed Alaskar, Associate Dr Farha Nazir, and Dr Fulwah Y Alqahtani from the College of Pharmacy, King Saud University.
Footnotes
Authors contribution
MQA is responsible for the study concept, data analysis, drafting, revising the article, gave final approval of the version to be published, and agrees to be accountable for all aspects of the work.
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Turner D, Little P, Raftery J, et al. Cost effectiveness of management strategies for urinary tract infections: results from randomised controlled trial. BMJ. 2010;340:c346. doi: 10.1136/bmj.c346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spencer JD, Schwaderer A, Mchugh K, Hains DS. Pediatric urinary tract infections: an analysis of hospitalizations, charges, and costs in the USA. Pediatr Nephrol. 2010;25(12):2469–2475. doi: 10.1007/s00467-010-1625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.François M, Hanslik T, Dervaux B, et al. The economic burden of urinary tract infections in women visiting general practices in France: a cross-sectional survey. BMC Health Serv Res. 2016;16(a):365. doi: 10.1186/s12913-016-1620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caterino JM, Weed SG, Espinola JA, Camargo CA. National trends in emergency department antibiotic prescribing for elders with urinary tract infection, 1996–2005. Acad Emerg Med. 2009;16(6):500–507. doi: 10.1111/j.1553-2712.2009.00353.x. [DOI] [PubMed] [Google Scholar]
- 5.Foxman B. Urinary tract infection syndromes. Infect Dis Clin North Am. 2014;28(1):1–13. doi: 10.1016/j.idc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Nawar EW, Niska RW, Xu J. National hospital ambulatory medical care survey: 2005 emergency department summary. Adv Data. 2007;386(386):1–32. [PubMed] [Google Scholar]
- 7.Martínez MA, Inglada L, Ochoa C, Villagrasa JR, Spanish Study Group On Antibiotic Treatments Assessment of antibiotic prescription in acute urinary tract infections in adults. J Infect. 2007;54(3):235–244. doi: 10.1016/j.jinf.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113(1A):5–13. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 9.Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. Vital Health Stat 13. 2011;169:1–38. [PubMed] [Google Scholar]
- 10.Foxman B, Barlow R, D’Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10(8):509–515. doi: 10.1016/s1047-2797(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J Antimicrob Chemother. 2014;69(1):234–240. doi: 10.1093/jac/dkt301. [DOI] [PubMed] [Google Scholar]
- 12.Wang KY, Seed P, Schofield P, Ibrahim S, Ashworth M. Which practices are high antibiotic prescribers? A cross-sectional analysis. Br J Gen Pract. 2009;59(567):e315–e320. doi: 10.3399/bjgp09X472593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi M, Shapiro DJ, Hersh AL, Sanchez GV, Hicks LA. Outpatient antibiotic prescribing practices for uncomplicated urinary tract infection in women in the United States, 2002–2011. Open Forum Infect Dis. 2016;3(3):ofw159. doi: 10.1093/ofid/ofw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO . The World Health Report 2007 A Safer Future: Global Public Health Security in the 21st Century. World Health Organization; 2007. [Google Scholar]
- 15.CDC Antibiotic Resistance Threats in the United States, 2013 [report on the Internet] [Accessed December 16, 2015]. p. 114. Available from: http://www.cdc.gov/dru-gresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf.
- 16.Bryce A, Hay AD, Lane IF, Thornton HV, Wootton M, Costelloe C. Global prevalence of antibiotic resistance in paediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: systematic review and meta-analysis. BMJ. 2016;352:i939. doi: 10.1136/bmj.i939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alanazi MQ, Al-Jeraisy MI, Salam M. Prevalence and predictors of antibiotic prescription errors in an emergency department, Central Saudi Arabia. Drug Healthc Patient Saf. 2015;7:103–111. doi: 10.2147/DHPS.S83770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Society of Health-System Pharmacists . AHFS Drug Information 2015. American Society of Health-System Pharmacists; 2015. [Accessed March 23, 2015]. Available from: http://www.ahfsdruginformation.com/drug-assignments.aspx. [Google Scholar]
- 19.Lexicomp . Drug Information Handbook. 24th ed. Lexicomp; 2014. [Accessed March 23, 2015]. Available from: http://www.lexi.com/home/revisions. [Google Scholar]
- 20.Freedman AL, Urologic Diseases in America Project Urologic diseases in North America Project: trends in resource utilization for urinary tract infections in children. J Urol. 2005;173(3):949–954. doi: 10.1097/01.ju.0000152092.03931.9a. [DOI] [PubMed] [Google Scholar]
- 21.Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008;27(4):302–308. doi: 10.1097/INF.0b013e31815e4122. [DOI] [PubMed] [Google Scholar]
- 22.Akbar DH. Urinary tract infection. Diabetics and non-diabetic patients. Saudi Med J. 2001;22(4):326–329. [PubMed] [Google Scholar]
- 23.Huang ES, Stafford RS. National patterns in the treatment of urinary tract infections in women by ambulatory care physicians. Arch Intern Med. 2002;162(1):41–47. doi: 10.1001/archinte.162.1.41. [DOI] [PubMed] [Google Scholar]
- 24.General Authority for Statistics . Statistical Yearbook of 2016. Kingdom of Saudi Arabia: General Authority for Statistics; 2016. [Accessed November 08, 2018]. Population. Available from: https://www.stats.gov.sa/en/169. [Google Scholar]
- 25.American College of Obstetricians and Gynecologists ACOG Practice Bulletin No. 91: Treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111(3):785–794. doi: 10.1097/AOG.0b013e318169f6ef. [DOI] [PubMed] [Google Scholar]
- 26.Garout WA, Kurdi HS, Shilli AH, Kari JA. Urinary tract infection in children younger than 5 years. Etiology and associated urological anomalies. Saudi Med J. 2015;36(4):497–501. doi: 10.15537/smj.2015.4.10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Ibrahim AA, Girdharilal RD, Jalal MA, Alghamdy AH, Ghazal YK. Urinary tract infection and vesicoureteral reflux in saudi children. Saudi J Kidney Dis Transpl. 2002;13(1):24–28. [PubMed] [Google Scholar]
- 28.Medina-Bombardó D, Seguí-Díaz M, Roca-Fusalba C, Llobera J, dysuria team What is the predictive value of urinary symptoms for diagnosing urinary tract infection in women? Fam Pract. 2003;20(2):103–107. doi: 10.1093/fampra/20.2.103. [DOI] [PubMed] [Google Scholar]
- 29.Wright OR, Safranek S. Urine dipstick for diagnosing urinary tract infection. Am Fam Physician. 2006;73(1):129–130. [PubMed] [Google Scholar]
- 30.Alanazi MQ, Alqahtani FY, Aleanizy FS. An evaluation of E. coli in urinary tract infection in emergency department at KAMC in Riyadh, Saudi Arabia: retrospective study. Ann Clin Microbiol Antimicrob. 2018;17(1):3. doi: 10.1186/s12941-018-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Tawfiq JA, Anani AA. Antimicrobial susceptibility pattern of bacterial pathogens causing urinary tract infections in a Saudi Arabian hospital. Chemotherapy. 2009;55(2):127–131. doi: 10.1159/000198698. [DOI] [PubMed] [Google Scholar]
- 32.Gobernado M, Valdés L, Alós JI, et al. Antimicrobial susceptibility of clinical Escherichia coli isolates from uncomplicated cystitis in women over a 1-year period in Spain. Rev Esp Quimioter. 2007;20(1):68–76. [PubMed] [Google Scholar]
- 33.Zhanel GG, Karlowsky JA, Harding GK, et al. A Canadian national surveillance study of urinary tract isolates from outpatients: comparison of the activities of trimethoprim-sulfamethoxazole, ampicillin, mecillinam, nitrofurantoin, and ciprofloxacin. The Canadian Urinary Isolate Study Group. Antimicrob Agents Chemother. 2000;44(4):1089–1092. doi: 10.1128/aac.44.4.1089-1092.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta K, Sahm DF, Mayfield D, Stamm WE. Antimicrobial resistance among uropathogens that cause community-acquired urinary tract infections in women: a nationwide analysis. Clin Infect Dis. 2001;33(1):89–94. doi: 10.1086/320880. [DOI] [PubMed] [Google Scholar]
- 35.Gupta K, Scholes D, Stamm WE. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA. 1999;281(8):736–738. doi: 10.1001/jama.281.8.736. [DOI] [PubMed] [Google Scholar]
- 36.Hadjipanayis A, Grossman Z, del Torso S, et al. Current primary care management of children aged 1–36 months with urinary tract infections in Europe: large scale survey of paediatric practice. Arch Dis Child. 2015;100(4):341–347. doi: 10.1136/archdischild-2014-306119. [DOI] [PubMed] [Google Scholar]
- 37.Canbaz S, Peksen Y, Tevfik Sunter A, Leblebicioglu H, Sunbul M. Antibiotic prescribing and urinary tract infection. Int J Antimicrob Agents. 2002;20(6):407–411. doi: 10.1016/s0924-8579(02)00252-2. [DOI] [PubMed] [Google Scholar]
- 38.Ironmonger D, Edeghere O, Gossain S, Hawkey PM. Use of antimicrobial resistance information and prescribing guidance for management of urinary tract infections: survey of general practitioners in the West Midlands. BMC Infect Dis. 2016;16:226. doi: 10.1186/s12879-016-1559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villani P, Demet D, Ambrosi P, Brondino-Riquier R, Bouvenot G. Diagnostic and therapeutic management of urinary infections. Survey in the medical services of the hospitals south of the Marseille Hospital Center. Presse Med. 2001;30(24 Pt 1):1204–1208. [PubMed] [Google Scholar]
- 40.Arnaud I, Elkouri D, N’Guyen JM, et al. Adequate prescription of antibiotic therapy for urinary tract infections in hospital: identifying and correcting non-observance of guidelines. Med Mal Infect. 2005;35(3):141–148. doi: 10.1016/j.medmal.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Hummers-Pradier E, Ohse AM, Koch M, Heizmann WR, Kochen MM. Management of urinary tract infections in female general practice patients. Fam Pract. 2005;22(1):71–77. doi: 10.1093/fampra/cmh720. [DOI] [PubMed] [Google Scholar]
- 42.Mclaughlin SP, Carson CC. Urinary tract infections in women. Med Clin North Am. 2004;88(2):417–429. doi: 10.1016/S0025-7125(03)00148-2. [DOI] [PubMed] [Google Scholar]
- 43.Ciani O, Grassi D, Tarricone R. An economic perspective on urinary tract infection: the “costs of resignation”. Clin Drug Investig. 2013;33(4):255–261. doi: 10.1007/s40261-013-0069-x. [DOI] [PubMed] [Google Scholar]