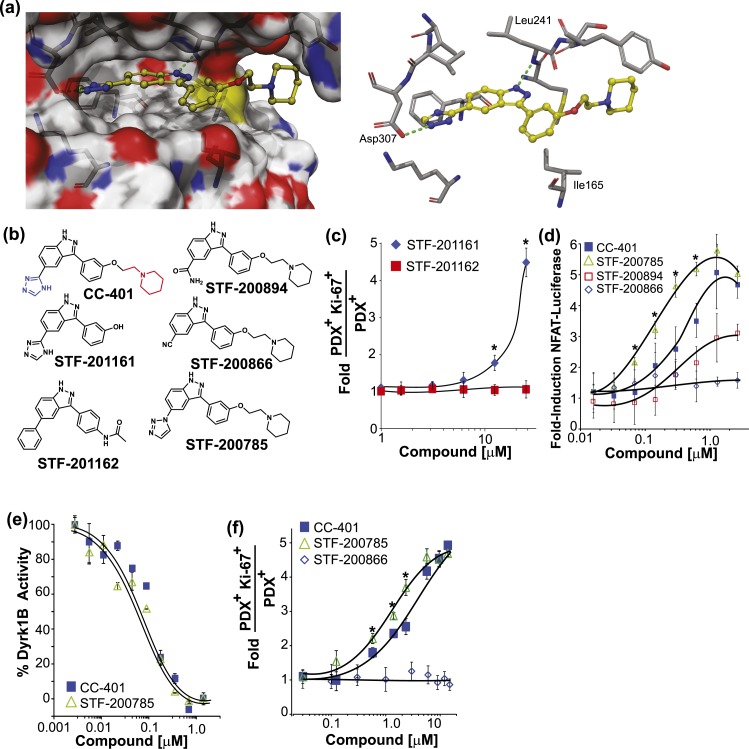
Figure 4.
Rationally designed CC-401 derivatives confirm DYRK1A/B as the relevant β-cell–replication target. (a) Space-filling (left) and stick (right) models of CC-401 binding to DYRK1A [4MQ2; Protein Data Bank (28)] generated with Schrodinger Glide software. Key hydrogen bond interactions are highlighted (green dashed line). (b) Chemical structures of CC-401 and synthesized derivatives. The piperidine group (red) and the triazole group (blue) are highlighted on CC-401. (c) Rat β-cell–proliferation response to STF-201161 and STF-201162. Data are presented as mean ± standard deviation (SD; n = 4 wells per treatment condition). *P < 0.05. (d) Fold-induction of NFAT-luciferase reporter activity in cells cotransfected with NFATc1, DYRK1A, and Renilla luciferase. Cells were compound treated as indicated. Data are presented as mean ± SD (n = 4 wells per experimental point). *P < 0.01 shown for STF-785 vs CC-401. A significant (P < 0.05) luciferase induction above DMSO occurred in response to CC-401 at a concentration >0.1 µM, STF-200785 (>0.05 µM), and STF-200894 (>2.0 µM). No luciferase induction occurred in response to STF-200866. (e) Recombinant DYRK1B activity measured in the presence of CC-401 or STF-200785 (n = 2 wells per experimental point). *P < 0.01. Half maximal inhibitory concentrations were not statistically different. (f) Fold increase in rat β-cell replication in response to the indicated compound treatment (n = 4 wells per treatment condition presented as mean ± SD). *P < 0.05 shown for STF-785 vs CC-401. No replication response occurred in response to STF-200866. Error bars represent the standard deviation of an experimental condition (n ≥ 3).