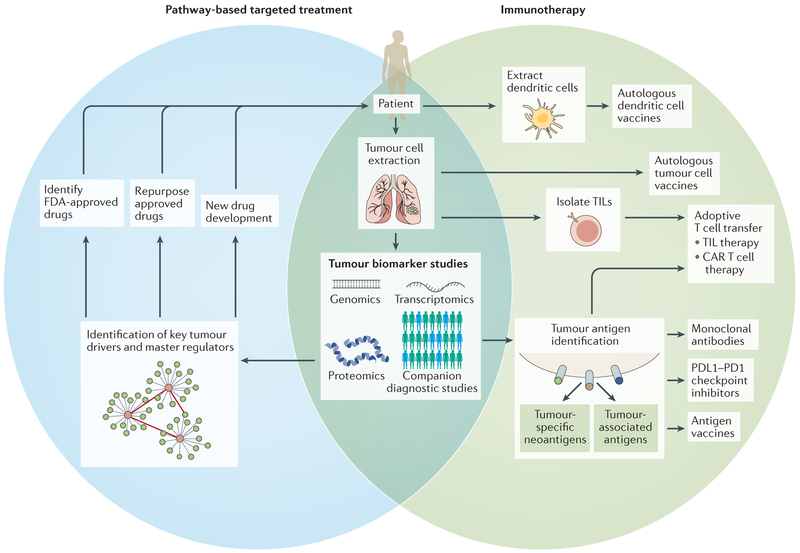
Figure 1 |. Precision therapy approaches in oncology.
Precision therapies in cancer generally use two primary approaches: pathway-based targeted therapies and immunotherapies. For both approaches, access to tumour cells (through resection or biopsy of solid tumours, or blood sample for haematological cancers or circulating tumour cells) enables an investigation into tumour biomarkers using various tools, including companion diagnostics, next-generation sequencing, gene expression profiling and proteomics. For pathway-based targeted treatments, these biomarker studies are used for the discovery of key drivers and master regulators of networks and pathways that promote tumour proliferation and survival. US Food and Drug Administration (FDA)-approved drugs that target these particular pathways can then be identified, or opportunities for drug repurposing or development may be explored for new targets. Alternatively, precision immunotherapy approaches include cell-based therapies, vaccines and biologics. Autologous (patient-derived) tumour cell and dendritic cell vaccines are generated from extracted tumours and dendritic cells, respectively. Extracted tumours may also be used to isolate tumour-infiltrating lymphocytes (TILs). This, in combination with tumour antigen data obtained from biomarker studies, has given rise to TIL-based adoptive cell therapies and chimeric antigen receptor (CAR) T cell therapies. The identification of tumour antigens, such as tumour-specific neoantigens or tumour-associated antigens, is also important for other personalized therapies, including antigen vaccines, programmed cell death 1 ligand 1 (PDL1)–PD1 checkpoint inhibitors and other monoclonal antibodies aimed at targeting tumour-promoting antigens.