Figure 1.
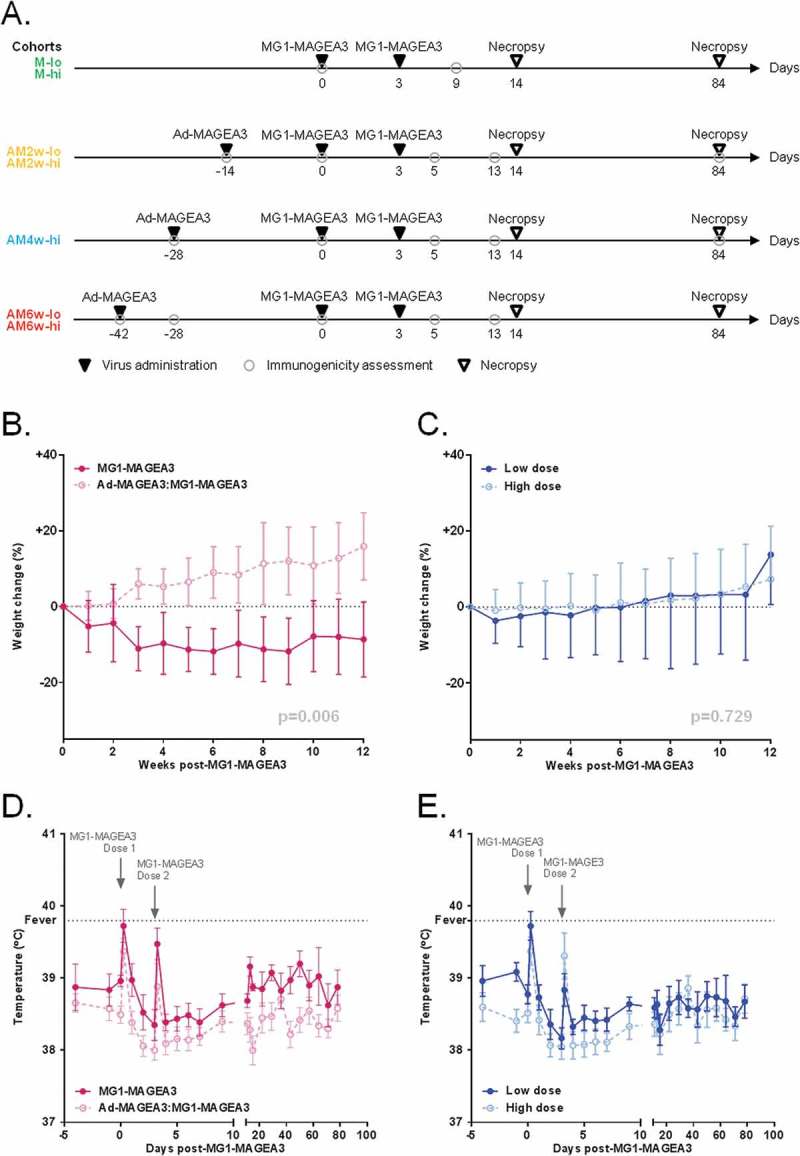
Ad-MAGEA3 and MG1-MAGEA3 are well tolerated by non-human primates.
(A) Study timeline in cynomolgus macaques. MG1-MAGEA3 was infused twice (3 days apart) at low (1 x 1010 PFU; ‘lo”) or high (1 x 1011 PFU; “hi”) doses, either as a standalone treatment (cohorts M-lo and M-hi; n = 4 each) or sequentially after receiving one intramuscular injection of 1 × 1010 PFU Ad-MAGEA3. For the latter setting, three intervals between Ad-MAGEA3 and MG1-MAGEA3 injections were tested: 2 weeks (cohorts AM2w-lo and AM2w-hi; n = 4 each), 4 weeks (cohort AM4w-hi; n = 4) or 6 weeks (cohorts AM6w-lo and AM6w-hi; n = 4 each). Blood samples were collected at the indicated time-points for immunogenicity assessments. Of note, the AM6w cohorts were dedicated to assessing the immunogenicity of the Ad-MAGEA3:MG1-MAGEA3 prime-boost and were not enrolled in the toxicology study. Body weight change relative to body weight at day 0 (cohorts M, AM2w and AM4w; n = 20) (B,C) and rectal temperatures (all macaques; n = 28) (D,E) by treatment arm (MG1-MAGEA3 alone versus Ad-MAGEA3:MG1-MAGEA3 regardless of the interval) or MG1-MAGEA3 dose (low dose versus high dose regardless of the treatment arm). Fever was classified as a rectal temperature ≥ 39.8°C (indicated by the horizontal dashed line). Body weight change is illustrated as mean ± SD and temperature as mean ± SEM. p-values (linear mixed models) are indicated and were considered significant when p < 0.05. MAGE-A3, human melanoma-associated antigen, family A, member 3; PFU, plaque-forming unit.
