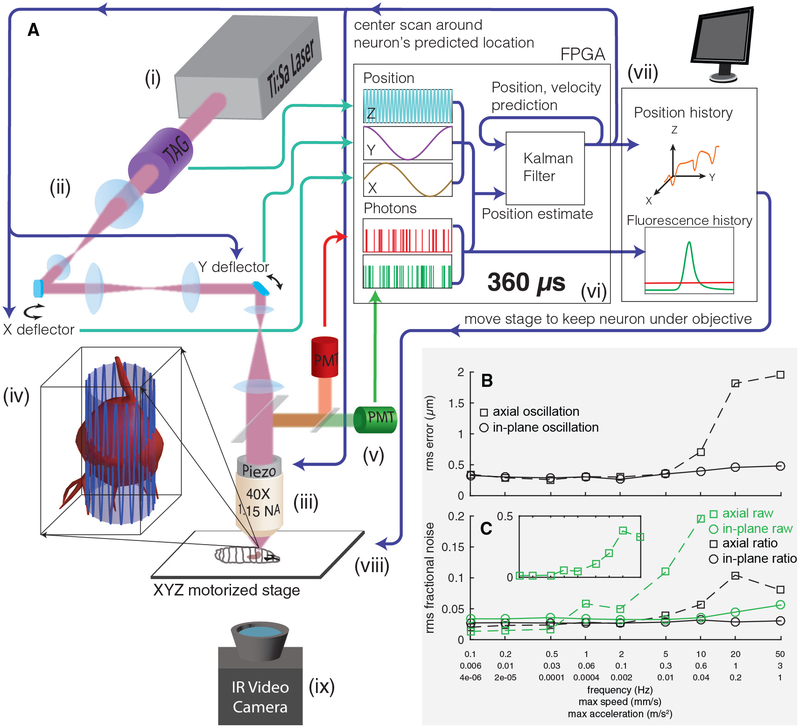
Figure 1. Tracking a Moving Neuron.
(A) Schematic of apparatus. Pulsed infrared excitation light is provided by a Ti:Sa laser (i). Scan optics consisting of two galvanometric mirrors and a resonant ultrasonic lens (ii) shape a wavefront that is relayed on to the back aperture of a piezo-mounted 40× objective (iii), scanning the two-photon excitation focal spot in a cylindrical pattern about a targeted neuron (iv). The scan pattern, schematized by the lightly shaded cylinder and oscillating blue line, has a diameter (7–8 μm) ~75% of that of the targeted neuron. The height of the cylinder (37 μm) has been shortened and the number of z oscillations reduced for clarity. Red and green fluorescence emission is captured by separate photomultiplier tubes (v). A field-programmable gate array (FPGA) (vi) correlates photon emission with focal spot position to create an estimate of the neuron’s location. A Kalman filter integrates all previous estimates to produce a prediction of the neuron’s position and velocity. The predicted center location is updated every 360 μs, and each cylindrical scan is centered on the neuron’s newly predicted location. The predicted position of the neuron and the number of red and green photons counted are sent to a computer (vii), which records data to disk for later analysis and controls a 3-axis stage (viii) to return the neuron to the natural center of the imaging system. A low-magnification infrared (IR) video camera placed below the stage (ix) records the posture of the larva for behavioral analysis.
(B and C) Tracking error (B) and noise in fluorescence (C) versus speed and acceleration. A single neuron was tracked while being oscillated in a 20 μm peak-to-peak sinusoidal wave at varying frequencies, either in the focal plane or axially. Note the logarithmic x axis, which is labeled with the frequency of oscillation (f) as well as the maximum speed (=A ∗ 2πf) and maximum acceleration (=A ∗ (2πf)2). A = 10 μm for all oscillations. See also Figure S1.