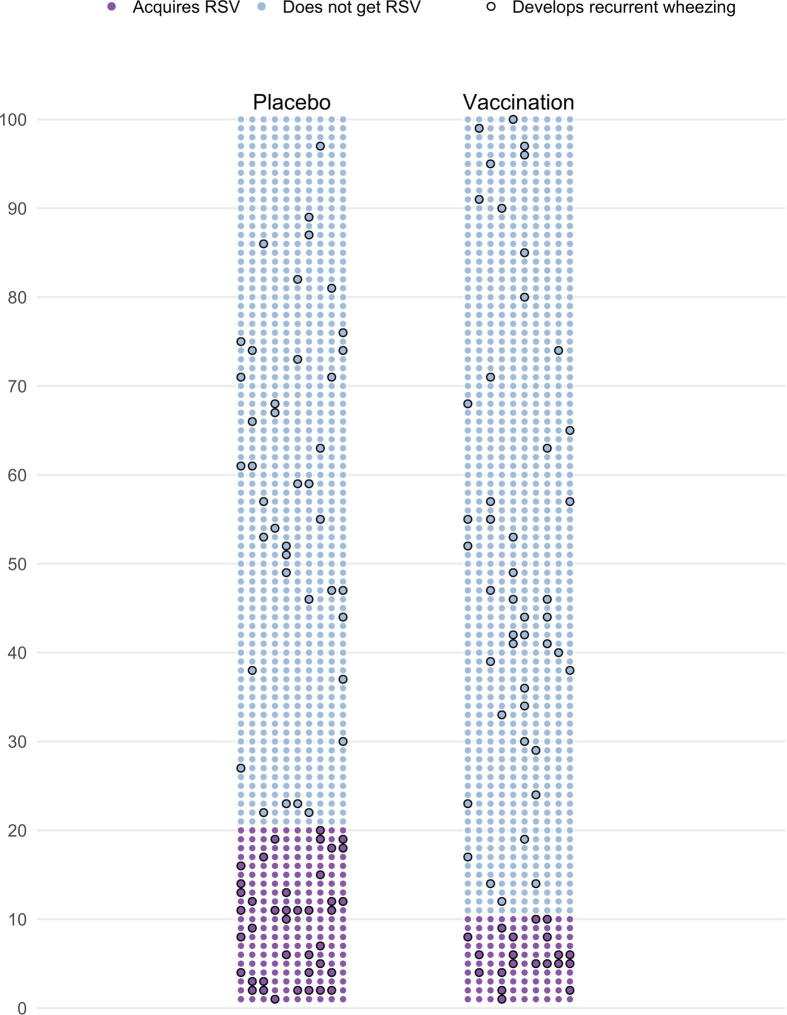
Fig. 2.
Illustration of the parameters used to estimate risk ratios and sample size in clinical trials of maternal RSV immunization on development of later recurrent childhood wheezing. Each filled dot in this figure represents a mother-infant pair, with the colour representing their RSV infection status and black outline indicating infants who go on to develop recurrent childhood wheezing. There are 100 rows of 10 dots, to represent 1000 mother-infant pairs randomized each to placebo and vaccination. Following the in-text example, 20% of placebo (200 mother-infant pairs; purple dots) acquire early and severe infant RSV vs. 10% in the immunized arm (100 mother infant pairs; purple dots), for a vaccine efficacy of 50%. If early RSV illness increases the later development of recurrent childhood wheezing, then the proportion of children who develop recurrent wheezing will be higher among those with early RSV. This is shown by the higher density of recurrent wheezing cases (black outline) among those with RSV illness (purple dots) vs. those without (blue dots). Summing the wheezing cases, there are 60 cases among the 300 infants who acquired RSV (for a 20% risk) vs. 85 wheezing cases among the 1700 infants who did not acquire RSV (for a 5% risk) giving rise to four-fold increased risk of wheezing in children exposed vs. unexposed to early and severe infant RSV. This example shows 80 cases of childhood recurrent wheezing among the placebo arm vs. 65 among the immunized arm for a risk ratio between vaccination and childhood recurrent wheezing (RRVW) of 0·81.