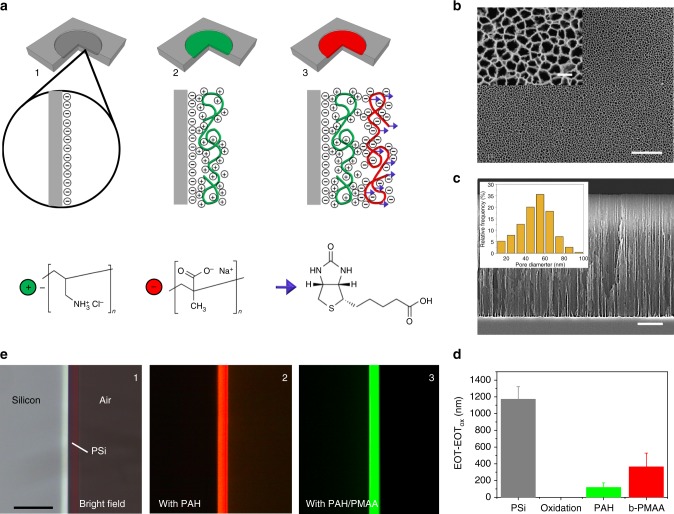
Fig. 2.
Layer-by-Layer biofunctionalization of nanostructured PSi interferometers. a Sketch of the biofunctionalization of the nanostructured surface of PSi interferometers via LbL nano-assembly: (1) preparation and oxidation of PSi interferometers (gray, negatively charged); (2) PAH coating (green, positively charged) of oxidized PSi interferometers; (3) b-PMAA (biotinylated PMMA) coating (red, negatively charged) of PSi/PAH interferometers. Details of the chemical structure of the polyelectrolytes used for LbL assembly, namely PAH (green circle) and PMAA (red circle), and of biotin (violet triangle) covalently linked to PMAA by amino-coupling, are given at the bottom of a. b SEM top-view image (50,000 × magnification) of the PSi interferometer surface (scale bar is 1.00 µm). The inset shows a higher magnification SEM image (200,000 × magnification) of the PSi surface that allows pore arrangement and size to be clearly appreciated (scale bar is 100 nm). c SEM cross-section image (25000 × magnification) of the PSi interferometer highlighting the columnar structure of the pores (scale bar is 1.00 μm). The inset shows a histogram of the pore size distribution, highlighting an average diameter of about 55 nm. d EOT-EOTox values recorded for PSi interferometers both as-prepared and after each LbL biofunctionalization step. The EOT value of oxidized PSi interferometers (i.e., EOTox) is used as reference to obtain positive differential EOT values. Data are provided as average values over seven replicates with error bars representing one standard deviation. e Bright-field optical image (1) and fluorescence images (2, 3) of the cross-section of oxidized PSi interferometers LbL-coated with (2) sulfo-rhodamine-labeled PAH and with (3) fluorescein-labeled PMAA on top of non-labeled PAH coating (scale bar is 15 µm). The homogeneity of the electrostatically driven LbL coating can be easily appreciated by the uniform fluorescence emission of the labeled polyelectrolytes over the whole PSi thickness