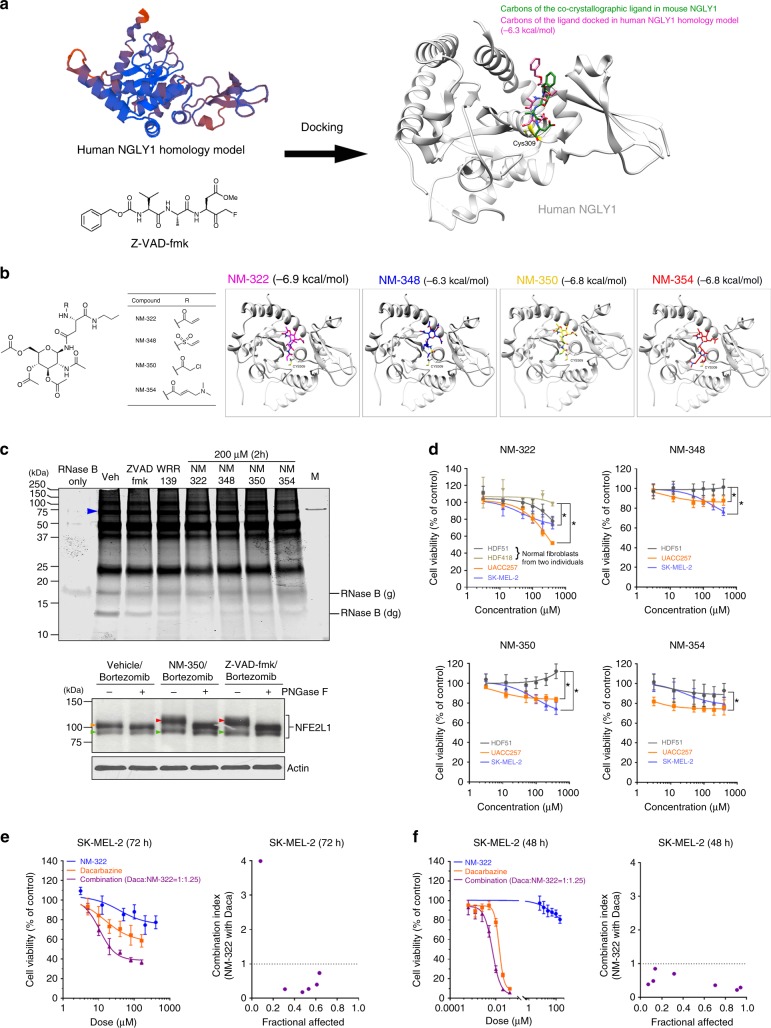
Fig. 6.
Anticancer responses induced by novel covalent modifiers that target the catalytic site of human NGLY1 in melanoma cells. The computational homology model of human NGLY1 core domain was generated and used for studying interactions between NGLY1 and novel small molecules that are designed to covalently modified and inactivate the catalytic site of NGLY1. (a) The most favourable binding pose of Z-VAD-fmk, a short peptide with NGLY1 and caspase inhibitory activity, in the human NGLY1 homology model superimposed to the conformation of Z-VAD-fmk bound to mouse NGLY1 in a co-crystalised structure. (b) Novel small molecules (NM-322, NM-348, NM-350, and NM-354) that mimic a GlcNAc-conjugated asparagine in the NGLY1 substrates of NGLY1 and contain strategically positioned electrophilic groups bound to the human NGLY1 homology model in computational docking and showed their high binding affinities with the electrophilic groups pointed towards Cys309 in close proximity at the human NGLY1 catalytic site. (c) Upper panel: the 2-hour reaction of covalent modifiers, including Z-VAD-fmk (20 µM), WRR139 (5 µM), NM-322, NM-348, NM-350 and NM-354, with human NGLY1 suppressed its activity in the deglycosylation of denatured RNase B. Blue arrowhead: recombinant NGLY1-FLAG. RNase B (g): glycosylated RNase B. RNase B (dg): deglycosylated RNase B. Veh: vehicle (DMSO) treatment. M: molecular weight marker. Lower panel: the deglycosylation of NFE2L1 altered by the treatment of 20 µM Z-VAD-fmk and 200 µM NM-350 in bortezomib-treated HEK293T cells. The cells were pretreated with vehicle (DMSO), Z-VAD-fmk and NM-350 for 24 h and subsequently subjected to concomitant treatment with 10 µM bortezomib for an additional 16 h. Cell lysates reacted with and without 500 units of PNGase F for 2 h were analysed using western blotting. Red arrowhead: fully glycosylated NFE2L1. Orange arrowhead: partially glycosylated NFE2L1. Green arrowhead: deglycosylated and truncated NFE2L1. (d) The dose-dependent suppression of cell viability was preferentially induced by the novel NGLY1 inhibitors in melanoma cells compared with normal cells (*P < 0.05, logistic regression). (e) The synergistic effect was observed between NM-322 and dacarbazine in the suppression of melanoma cell viability. The cell viability curve of combinatorial treatment was plotted according to the doses of dacarbazine used in the treatment. (f) The synergistic effect was observed between NM-350 and bortezomib in the suppression of melanoma cell viability. The cell viability curve of combinatorial treatment was plotted according to the doses of bortezomib used in the treatment. All the data of cell viability tests were presented as mean ± standard deviation (n = 3)