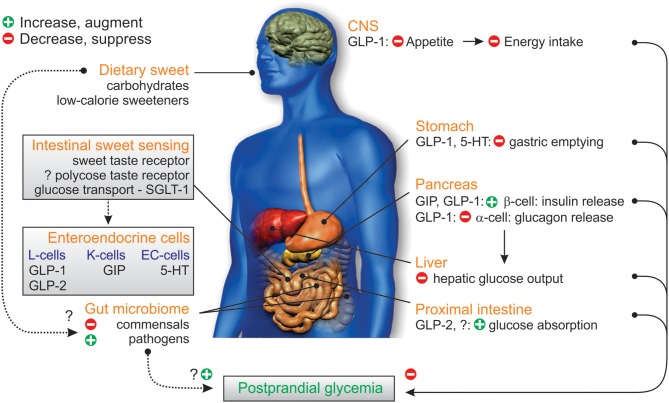
Figure 3.
Gastrointestinal factors influencing glycemic control. Dietary sweet stimuli can activate STR in the proximal intestine facilitating the enteroendocrine cell release of the incretin peptides GIP from K-cells and GLP-1 from L-cells, as well as 5-HT from EC-cells; substrates of SGLT-1 (glucose, galactose) also trigger GIP and GLP-2 release. GIP and GLP-1 stimulate glucose-dependent insulin release, to increase glucose disposal; GLP-1 and 5-HT also slow the rate of gastric emptying via vagus nerve signals (not shown) while GLP-1 inhibits pancreatic glucagon release, leading to reduced hepatic glucose output. GLP-2 co-released from L-cells acts to increase intestinal glucose absorption via an increase in the capacity for SGLT-1-based glucose transport. Dietary sweet stimuli can also alter the composition of the gut microbiome in favor of colonization of gut pathogens over fermentative gut commensals, which can affect energy harvest, and disrupt microbiome signaling to the host and glycemic control. Together these influences can disrupt the homeostatic balance between glucose-evoked gut hormone release, glucose absorption, and microbiome composition, leading to dysglycemia which would potentially be harmful in the setting of type 2 diabetes. In addition, complex carbohydrates (oligosaccharides) may contribute to these processes via a yet to be identified polycose taste receptor.