FIGURE 2.
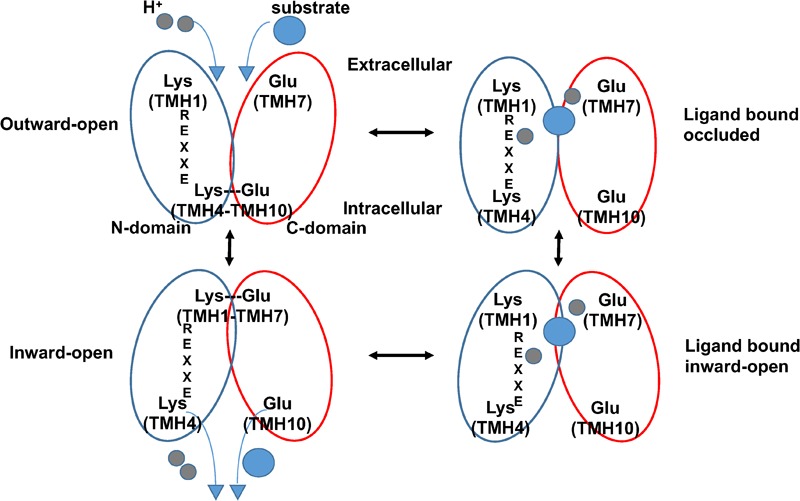
Proposed alternating-access mechanism in NPF/POT transporters. Schematic model of the proposed NPF/POT alternating-access transport cycle showing four conformations (based on Newstead, 2015). In the outward-open conformation, the transporter is open towards the extracellular space; a salt bridge forms between residues on TMH4 and TMH10, holding the N- and C-terminal bundles (blue and red ovals, respectively) together and stabilizing the conformation. Negatively charged amino acids in TMH7 and in the ExxER/K motif on TMH1 are exposed in the internal cavity and are available for proton binding. After protons (gray spheres) and substrate (blue sphere) bind, the protein undergoes conformational changes that include the disruption of the TMH4–TMH10 salt bridge allowing the transporter to open towards the intracellular space in the inward-open conformation. Finally, protons and substrate are released in the cytoplasm. A salt bridge may form in the inward-open conformation between oppositely charged residues on TMH1 and TMH7 as observed in some crystal structures from bacterial POTs.
