Figure 1.
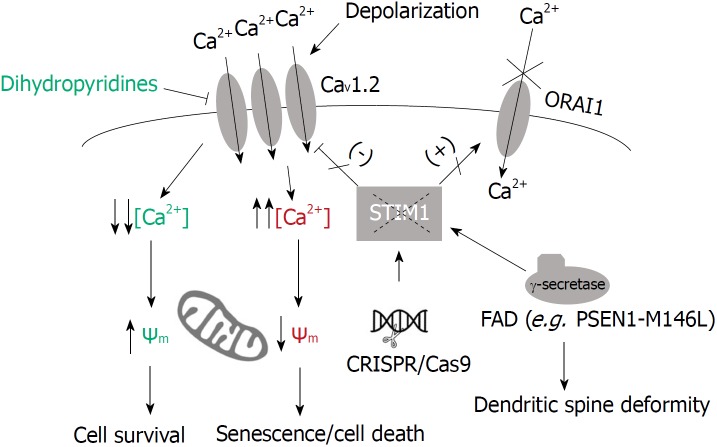
Deficiency of STIM1 and neurodegeneration. Neurons expressing the mutant PSEN1 M146L showed higher rates of STIM1 proteolysis, reduced levels of STIM1, reduced store-operated Ca2+ entry and diminished dendritic spines[46]. Deficiency of STIM1 has been observed in non-familial (sporadic) Alzheimer’s disease (AD) patients, and can be mimicked by genome edition of STIM1 locus in SH-SY5Y cells[47]. Because STIM1 is a negative regulator of CaV1.2 channels, this deficiency triggered the upregulation of Ca2+ entry through CaV1.2 channels which was responsible for the loss of inner mitochondrial membrane polarization, senescence, and cell death[47]. This higher rate of Ca2+ influx through CaV1.2 channels has also been monitored in 3xTgAD mice[51]. The long-term treatment with dihydropyridines, known blockers of CaV1.2, reduced sporadic dementia by 55% during aging[52], pointing out the decrease of STIM1 as a possible mechanism to explain neurodegeneration in sporadic and familial AD.