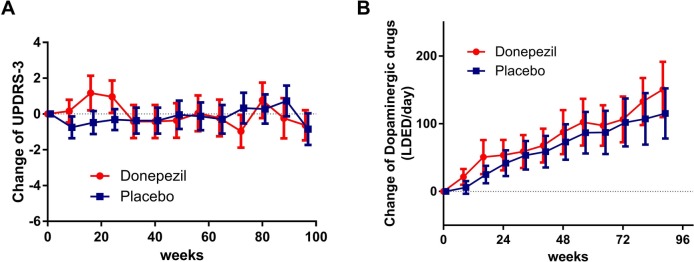
Figure 4.
Changes in UPDRS-III scores and L-Dopa equivalent dose of dopaminergic replacement therapy (LDED). (A) In the donepezil group, UPDRS-III scores were transiently elevated by starting and dose escalation (3–5 mg/day) of donepezil, but then returned to baseline and remained stable. In the placebo group, the scores were stable. There were no statistically significant differences in UDPRS-III changes between donepezil and placebo groups. (B) Dopaminergic drug doses were elevated gradually in both donepezil and placebo groups and were slightly larger with donepezil than with placebo. The difference was not statistically significant. Data represent the mean with 95% CIs.