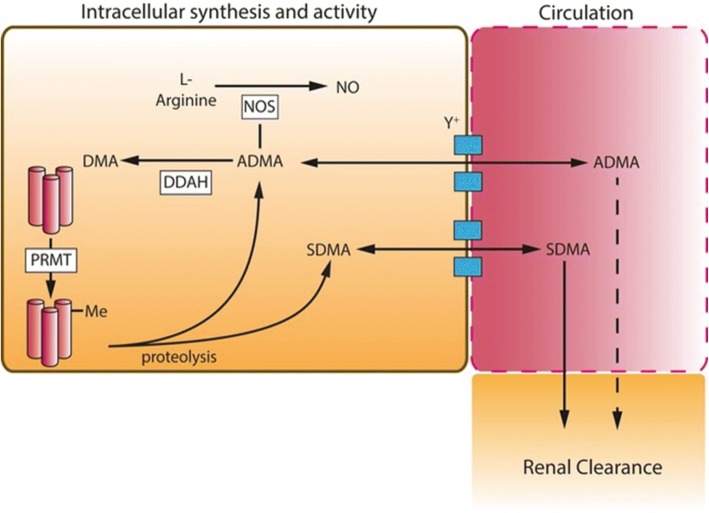
Fig. 1.
Representative image of the synthesis and regulation of asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA) in sepsis. Protein arginine methyl transferases (PRMT) catalyse the methylation of arginine containing protein residues to ADMA and SDMA, which are released upon proteolysis. ADMA and SDMA are transported via the y+ cationic amino acid transporter into and out of the circulation. SDMA is not metabolised in most cell,s whereas ADMA is metabolised by the two isoforms of dimethylarginine dimethylaminohydrolase (DDAH) in a wide range of tissues. ADMA acts intracellularly to inhibit nitric oxide synthase (NOS), SDMA has no action on NOS isoforms. SDMA is cleared by the kidney largely unchanged and in small part through metabolism by AGXT2 (not shown). ADMA is largely metabolised by DDAH to dimethylamine (DMA); a small amount is cleared unchanged through the kidney. In sepsis, the synthesis of ADMA and SDMA may be increased through high protein-turnover in patients in a catabolic state and reduced renal clearance as a consequence of acute kidney injury. Differences in the relative concentrations of these methylarginines reflect their differential metabolism by DDAH isoforms as only ADMA is a DDAH substrate. Note, monomethylarginine (L-NMMA) is considered to have the same synthetic pathway, activity, metabolism and clearance as ADMA but is present in only 10% of the concentration