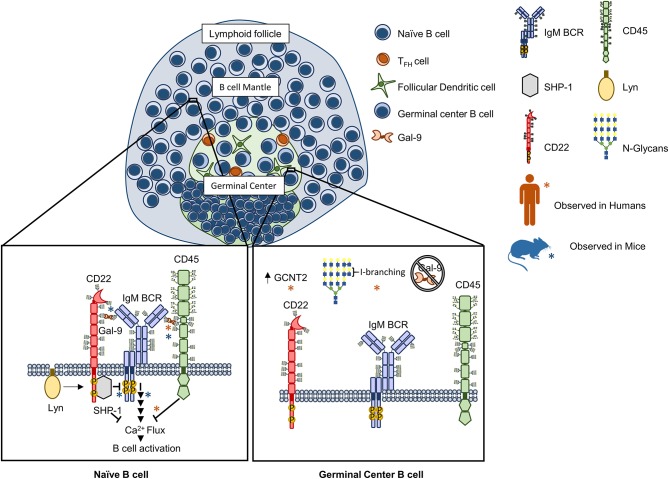
Figure 1.
Galectin-9 regulates B cell receptor signaling in human and murine B cells through analogous but distinct mechanisms. Naïve B cell activation is antagonized by Gal-9 through binding to the receptor tyrosine phosphatase CD45 on human B cells that activates a Lyn-CD22-SHP-1 dependent circuit and inhibits calcium accumulation downstream of the BCR [Left, orange asterisks (*)]. In murine B cells, Gal-9 similarly regulates BCR signaling by altering the nanoscale organization of signaling molecules. Specifically, Gal-9 has been found to bind CD45 but also IgM BCR, preventing exclusion of CD45 and CD22 upon B cell activation and leading to impaired signal transduction following BCR ligation [Left, blue asterisks (*)]. In humans, Gal-9 binding activity is differentially regulated between naïve and germinal center (GC) B cells via concerted alterations in N-glycosylation. Specifically, Gal-9 binding has been shown to be greatly diminished in germinal center (GC) B cells via upregulation of the glycosyltransferase GCNT2, which catalyzes I-branch formation on glycan ligands of Gal-9 (poly-LacNAcs), and attenuates Gal-9 binding (56, 57).