Abstract
Background
Outcome data for infants on chronic peritoneal dialysis (CPD) is limited and has been based primarily on the analyses of voluntary entry registry data. In contrast, the United States Renal Data Systems (USRDS) collects data on all infants with end-stage kidney disease (ESKD) on chronic dialysis in the USA. We aimed to describe the clinical characteristics of this population and to determine the associated patient mortality.
Methods
The USRDS database was reviewed retrospectively for data on infants who initiated CPD at ≤ 12 months of age from 1990 to 2014. Infants were categorized into four groups, CPD initiation age (≤ 1 month of age or neonates and > 1–12 months of age or older infants) and initiation era (1990–1999 and 2000–2014).
Results
A total of 1723 infants (574 neonates and 1149 older infants) were identified. Overall, 20.9% of infants (147 neonates and 213 older infants) died on dialysis during the follow-up. The most commonly identified causes of death on dialysis were cardiorespiratory disease (25.8%) and infection (22.8%). There was an increased risk for mortality in all infants who initiated CPD in the earlier initiation era (1990–1999) vs the later era (2000–2014) (aHR of 1.95), for females vs males (aHR 1.43), and for those with a primary diagnosis of cystic kidney diseases vs congenital anomalies of the kidney and urinary tract (CAKUT) (aHR 1.84). In 2000–2014, patient survival at 1 and 5 years was 86.8% and 74.6% for those who initiated CPD as neonates and 89.6% and 79.3% for those who did so as older infants.
Conclusions
In this large cohort of infants who received chronic peritoneal dialysis over more than two decades, the probability of survival after initiating CPD in the first year of life has significantly improved. There is no difference in the probability of death for neonates compared to older infants. However, the mortality rate remains substantial in association with multiple risk factors.
Keywords: Peritoneal dialysis, Chronic peritoneal dialysis, Pediatric ESKD, Infants, Neonates
Introduction
The development of end-stage kidney disease (ESKD) in an infant (< 12 months of age) is a rare occurrence. In the USA, although children 0–4 years of age account for approximately 23% of all children on chronic peritoneal dialysis (CPD), the incidence of ESKD in the first year of life is only 10 cases per million population [1–3]. Additional incidence data is available from the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) registry which showed an ESKD incidence of 7.1 cases per million age-related population in infants aged 0–2 years, and from Carey et al. who have estimated the incidence of ESKD in neonates to be only 0.32 cases per million live births [4, 5].
The limited number of infants with ESKD has most importantly compromised the ability to obtain substantial outcome data to inform providers and affected families. Moreover, historical reports from single centers and voluntary registries have provided dismal outcomes for infants relative to older dialysis patients [2, 5–9]. Single-center data have reported 1- and 5-year survival for neonates on CPD to be as low as 40–50% [5, 6]. However, more recent data have suggested that outcomes have improved for infants who have received chronic dialysis therapy [10–12]. A recent analysis based on data from the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) registry evaluated the outcomes of 241 neonates (< 31 days) and 387 older infants (> 1–12 months) who initiated CPD over several decades [13]. This analysis revealed improved outcomes over time for these patients with respect to access to transplantation and risk of mortality. Nevertheless, the voluntary nature of the NAPRTCS database, as well as other single-center or voluntary registries, was acknowledged by the authors to be an important limitation of their analysis by potentially leaving room for selection bias and associated inflated outcome data [14].
The United States Renal Data System (USRDS) has collected information on virtually all children diagnosed with ESKD who have received chronic dialysis in the United States (US) since 1990. In turn, the database provides the information necessary to more completely describe current and historical treatment and outcome data on neonates and infants with ESKD who have received chronic renal replacement therapy (RRT) in contrast to the published voluntary data. Based on prior data published by the NAPRTCS that chronic hemodialysis is prescribed for only a small percentage (< 3%) of infants < 1 year of age with ESKD, the objective of this study was to describe demographic features and patient survival data for all US infants diagnosed with ESKD and initiated on CPD within the first year of life based on data derived from the USRDS from 1990 to 2014 [4, 13, 15, 16]. We hypothesized that as has been suggested by recent registry reports, patient survival has improved over time, particularly in younger infants.
Patients and methods
This was a retrospective cohort study of all infants ≤ 12 months of age at the time of initiation of CPD due to a diagnosis of ESKD during the interval of January 1, 1990, to December 31, 2014, as recorded in the USRDS database. To help facilitate this process, pediatric nephrologists in the US are required to complete a Centers for Medicare & Medicaid (CMS) Medical Evidence Report (CMS-2728) for all pediatric patients with a diagnosis of ESKD who are permanent residents or citizens of the US and receiving RRT. After initial entry of demographic information pertaining to dialysis into the database, follow-up data is also entered upon the date of death. Data on renal transplantation is reported to the United Network for Organ Sharing (UNOS) and collected in the USRDS database as well.
Patients
Infants ≤ 12 months of age at the time of initiation of chronic dialysis were included in the analysis if their primary mode of dialysis was listed as continuous cycling peritoneal dialysis (CCPD), continuous ambulatory peritoneal dialysis (CAPD), or other unspecified CPD modality. Infants were excluded from the analysis if their primary RRT modality was either hemodialysis or renal transplant.
Demographics
To evaluate demographic data separately for patients who initiated CPD during early and late infancy, we categorized children into those who began CPD at ≤ 1 month of age (neonates) and > 1–12 months of age (older infants), respectively. Demographic data included in the analysis consisted of the following: sex, race, ethnicity, primary renal disorder, initial CPD modality, and cause of death. The USRDS captures specific renal diagnoses and then groups the diagnoses into major categories. Some of the major diagnosis groups in the USRDS database are rarely seen in infants who receive chronic dialysis. Therefore, we individually reviewed the primary ESKD diagnosis of all patients in the cohort and regrouped them according to categories that were clinically relevant to the pediatric population based upon the most common diagnoses previously reported in the pediatric dialysis literature [4–6]. Similarly, the cause of death for each patient was individually reviewed and grouped into categories clinically relevant for pediatric patients. Age at dialysis initiation was provided and used to calculate time from dialysis to transplant and age at death, for comparison between the two age groups and two initiation eras. Median measures of central tendency with interquartile ranges were used to compare time on dialysis and time to transplant.
Outcomes
We evaluated the associations between patient survival while on dialysis with demographic data (race, sex, and primary ESKD diagnosis), age at initiation of CPD, and era of CPD initiation.
As the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) Clinical Practice Guidelines on peritoneal dialysis were first published in 1997 and began to include more pediatric-specific recommendations in the 2000 update, we hypothesized that notable changes in outcome would be seen before and after year 2000 [17, 18]. For this reason, we compared the outcome of infants by age at dialysis initiation (neonate vs. older infant) and era of initiation on CPD (1990–1999 and 2000–2014) over a 10-year period. Based upon previously published survival data of children on dialysis, we assumed that all surviving pediatric patients would have undergone renal transplantation within a 10-year follow-up period from dialysis initiation. Patients were, in turn, censored at the time of transplant or last follow-up to January 1, 2015, and/or up to 10 years from dialysis initiation.
Statistical analysis
To identify patient characteristics associated with the cumulative incidence of death on dialysis, we completed a competing risk model (including 95% confidence intervals) similar to the Fine and Gray model [19, 20]. A competing risk is an event that modifies the chance that another event may occur. Our analysis considers death on dialysis as the primary event with kidney transplantation as the competing risk event resulting in cause-specific hazard ratios. This approach more appropriately weights patients who experience death considering the competing event of kidney transplantation in the assessment of survival. The final model was adjusted to evaluate the multivariate association between the probability of death and the demographic characteristics of initiation era, age group, race, and sex.
Pearson’s chi-square was used for comparisons of continuous variables. All tests of significance were two-sided with α level of confidence of 0.05. The analysis was completed with SAS version 9.4 using standard analysis files obtained from the USRDS. The study was approved by the Institutional Review Boards of Children’s Mercy Kansas City and the University of North Carolina (retrospective review).
Results
Patient characteristics
A total of 1723 infants were identified as being ≤ 12 months of age when CPD was initiated between January 1, 1990, and December 31, 2014. As shown in Table 1, the majority of the infants were non-Hispanic (73%), white (77%), and male (68%). The most common primary ESKD diagnosis was congenital anomalies of the kidney and urinary tract (CAKUT) (55%), and the most common initial CPD modality was continuous cycling peritoneal dialysis (CCPD) (85%).
Table 1.
Characteristics of infants initiated on chronic peritoneal dialysis (CPD) at age ≤ 12 months in the United States Renal Data System (USRDS), with data stratified by age at CPD initiation and era of initiation
| Era of initiation | 1990–1999 | 2000–2014 | ||||
|---|---|---|---|---|---|---|
| ≤ 1 month (N = 202) |
>1–12 months (N = 341) |
Totals (N = 543) |
≤ 1 month (N = 372) |
> 1–12 months (N = 808) |
Totals (N = 1180) |
|
| Sex, N (%) | ||||||
| Male | 140 (69.3) | 224 (65.7) | 364 | 263 (70.7) | 556 (68.9) | 819 |
| Female | 62 (30.7) | 117(34.3) | 179 | 109 (29.3) | 252 (31.1) | 361 |
| Race, N (%) | ||||||
| White | 159 (78.7) | 271 (79.5) | 430 | 293 (78.8) | 605 (74.9) | 898 |
| Black | 37 (18.3) | 52 (16.1) | 89 | 58 (15.6) | 144 (17.8) | 202 |
| Other | <10a | 15 (4.4) | 19 | 21 (5.6) | 58 (7.2) | 79 |
| Missing | <10a | <10a | <10a | 0 | <10a | <10a |
| Ethnicity, N (%) | ||||||
| Hispanic | 33 (16.3) | 66 (19.3) | 99 | 83 (22.3) | 188 (23.3) | 271 |
| Non-Hispanic | 140 (69.3) | 215 (63) | 355 | 288 (77.4) | 615 (76.1) | 903 |
| Other | 24 (11.9) | 51 (15) | 75 | 0 | 0 | 0 |
| Missing | <10a | <10a | <10a | <10a | <10a | <10a |
| Diagnosis, N (%) | ||||||
| CAKUT | 128 (63.4) | 174 (51) | 302 | 213 (57.3) | 434 (53.8) | 647 |
| Cystic kidney Disease | 31 (15.3) | 45 (13.2) | 76 | 54 (14.5) | 86 (10.6) | 140 |
| Other | 43 (21.3) | 120 (35.2) | 163 | 105 (28.2) | 278 (34.4) | 383 |
| Missing | 0 | <10a | <10a | 0 | 10(1.2) | 10 |
| Dialysis modality, N (%) | ||||||
| CAPD | 39 (19.3) | 73 (21.4) | 112 | 19(5.1) | 34 (4.2) | 53 |
| CCPD | 134 (66.3) | 233 (68.3) | 367 | 343 (92.2) | 760 (94.1) | 1103 |
| Other PD unspecified | 29 (14.4) | 35 (10.3) | 61 | 10 (2.7) | 14(1.7) | 24 |
CCPD continuous cycling peritoneal dialysis, CAPD continuous ambulatory peritoneal dialysis, CAKUT congenital anomalies of the kidney and urinary tract
For all values where N is less than 10, the data is required to be reported as an N < 10 per USRDS guidelines
Renal replacement therapy
The median age at dialysis initiation for all infants who started CPD in the prior initiation era (1990–1999) was 2.2 months (IQR 0.5–5.7 months), whereas the median age at dialysis initiation was 2.5 months (IQR 0.7–6.0 months) for the recent initiation era (2000–2014) (Table 2).
Table 2.
Median age at dialysis initiation and median age at renal transplantation for infants initiated on chronic peritoneal dialysis (CPD) at age ≤ 12 months, with data stratified by age at CPD initiation and era of initiation
| Era of initiation | 1990–1999 | 2000–2014 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤ 1 month | > 1–12 months | ≤ 1 month | > 1–12 months | |||||||||
| N | Median | IQR | N | Median | IQR | N | Median | IQR | N | Median | IQR | |
| Age at dialysis initiation (months) | 202 | 0.33 | 0.2–0.6 | 341 | 4.70 | 2.6–7.5 | 372 | 0.33 | 0.2–0.6 | 808 | 4.64 | 2.4–7.5 |
| Age at transplant (years) | 124 | 2.52 | 1.6–3.9 | 228 | 2.02 | 1.4–3.1 | 209 | 2.32 | 1.7–3.1 | 499 | 2.08 | 1.6–2.9 |
| Time from dialysis to transplant (years) | 124 | 2.49 | 1.6–3.9 | 228 | 1.49 | 0.9–2.7 | 209 | 2.27 | 1.7–3.0 | 499 | 1.61 | 1.1–2.4 |
Overall, 1060 (61.5%) of the 1723 patients received at least one transplant during the follow-up period. Median age at transplant was 2.2 years (IQR 1.5–3.5 years) vs 2.0 years (IQR 1.5–2.9 years), and time to transplant was 1.9 years (IQR 1.2–3.2 years) vs 1.8 years (IQR 1.2–2.6 years) for all infants who initiated dialysis in the 1990–1999 era compared to the 2000–2014 era of dialysis initiation, respectively (Table 2).
Survival trends
There were 360 (21%) patient deaths recorded during the initial course of dialysis in the entire cohort. Thirty-two percent of neonates and 28% of older infants who initiated dialysis during the prior era (1990–1999) died on dialysis prior to transplantation. In contrast, 22% of neonates and 14% of older infants who initiated CPD in the contemporary era died on dialysis prior to transplantation. Among those who died, the median age at death was 0.95 years (IQR 0.5–1.5 years) for all infants who initiated CPD from 1990 to 1999 and 0.97 years (IQR 0.6–1.4 years) for all infants who initiated dialysis in the more recent era. The median age of death by age and era of initiation is presented in Table 3.
Table 3.
Median age at death and primary cause of death during treatment with chronic peritoneal dialysis (CPD), with data stratified by age at CPD initiation and era of initiation
| 1990–1999 | 2000–2014 | |||||||
|---|---|---|---|---|---|---|---|---|
| ≤ 1 month (N =64) |
> 1–12 months (N =96) |
≤ 1 month (N = 83) |
> 1–12 months (N =117) |
|||||
| N (%) | N (%) | N (%) | N (%) | |||||
| Cardiorespiratory | 21 (32.8) | 25 (26) | 20 (24.1) | 27(23.1) | ||||
| Infection | 13 (20.3) | 21 (21.9) | 22 (26.5) | 26 (22.2) | ||||
| Withdrawal | 0 | 0 | <10a | <10a | ||||
| Other | 22 (34.3) | 28 (29.2) | 16 (19.2) | 24 (20.5) | ||||
| Missing | <10a | 22 (22.9) | 22 (26.5) | 35 (29.9) | ||||
| Median age at death (years) | Med | IQR | Med | IQR | Med | IQR | Med | IQR |
| 0.63 | 0.4–1.2 | 1.16 | 0.7–1.9 | 0.83 | 0.4–1.2 | 1.05 | 0.7–1.5 | |
Using Pearson’s chi-square, there was no statistically significant difference between causes of death by age group controlling for era of dialysis initiation (p = 0.74)
For all values where N is less than 10, the data is required to be reported as an N < 10 per USRDS guidelines
The primary reported cause of death was available for 273 of the 360 patients who died during their course on dialysis (Table 3). Overall, cardiorespiratory failure (25.8%) was the most common identified cause of death, followed by infection (22.8%). There was no statistically significant difference in the cause of death by age group or era of dialysis initiation (p = 0.74).
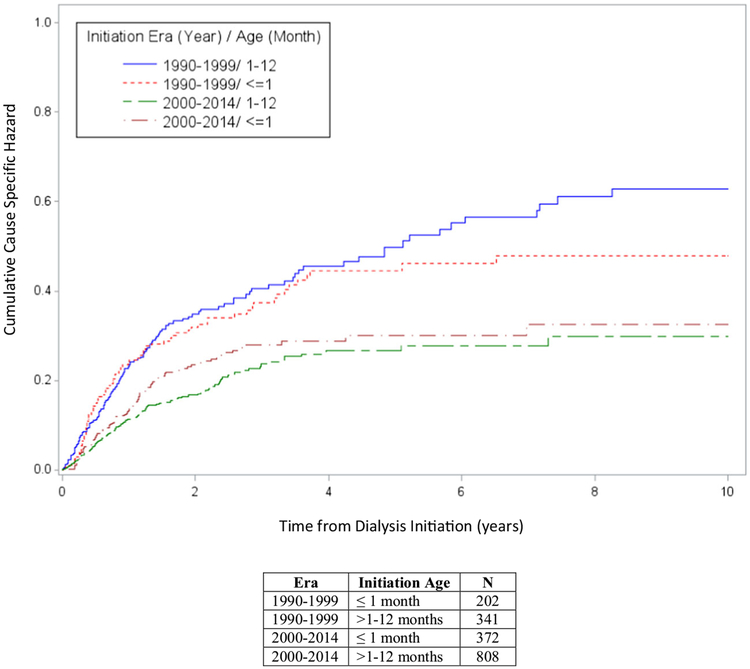
Figure 1 demonstrates the cause-specific cumulative incidence competing risk survival analysis in infants ≤ 1 month of age and > 1–12 months of age at CPD initiation by treatment era (1990–1999 and 2000–2014). Both patient age group cohorts who initiated CPD in 2000–2014 experienced better patient survival when compared to those who began dialysis in the prior era (p < 0.0001). Patient survival at 1 and 5 years was 86.8% and 74.6% for infants started on CPD at ≤ 1 month of age during dialysis initiation era 2000–2014 compared to 76.9% and 63.8% 1- and 5-year survival, respectively, during the era 1990–1999. Likewise, 1- and 5-year survival were 89.6% and 79.3% for infants > 1–12 months of age at CPD initiation during the 2000–2014 era, compared to 80.8% and 61.6% 1- and 5-year survival, respectively, between 1990 and 1999.
Fig. 1.
The cumulative cause-specific hazard plot of death on dialysis for infants initiated on chronic peritoneal dialysis (CPD) at age ≤ 12 months, stratified by age at CPD initiation and era of initiation
Adjusted associations with mortality
Taking into consideration the competing risk of transplantation, when evaluating for associations between patient characteristics and mortality, there was a statistically significant adjusted HR of 1.95 (95% CI 1.62–2.35, p < 0.0001) for initiation era (1990–1999 vs 2000–2014). There was also an increased risk of mortality in females initiating CPD compared to male infants initiating CPD (adjusted HR 1.43, 95%CI 1.18–1.74, p = 0.0003) in the final model (Table 4). There was, however, no significant difference in risk of mortality based on age of dialysis initiation and race.
Table 4.
The competing risk model of time to death on dialysis with transplantation as competing risk adjusted by demographic characteristics
| Variables | Comparison group | Unadjusted model | Adjusted modela | Final adjusted modelb | |||
|---|---|---|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | ||
| Initiation era | 1990–1999 vs. 2000–2014 | 1.95 (1.62–2.36) | <0.0001 | 1.89 (1.55–2.31) | < 0.0001 | 1.95 (1.62–2.35) | < 0.0001 |
| Age | ≤ 1 month vs. 1–12 months | 1.11 (0.92–1.34) | 0.2882 | 1.08(0.89–1.31) | 0.4563 | 1.07 (0.89–1.30) | 0.4645 |
| Race | Black vs. white | 1.02 (0.80–1.29) | 0.8862 | 1.13 (0.88–1.44) | 0.3429 | 1.05 (0.83–1.34) | 0.6706 |
| Other vs. white | 1.30 (0.91–1.86) | 0.1497 | 1.28 (0.89–1.86) | 0.1849 | 1.38 (0.96–1.97) | 0.0812 | |
| Sex | Female vs. male | 1.45 (1.19–1.75) | 0.0002 | 1.26 (1.03–1.54) | 0.0257 | 1.43 (1.18–1.74) | 0.0003 |
| Primary diagnosis | Cystic kidney diseases vs. CAKUT | 2.01 (1.56–2.60) | <0.001 | 1.84 (1.41–2.40) | < 0.0001 | − | − |
| Other vs. CAKUT | 1.41 (1.14–1.73) | 0.0013 | 1.36(1.09–1.69) | 0.0064 | |||
| Dialysis modality | CAPD vs. CCPD | 1.38 (1.04–1.83) | 0.0274 | 1.04(0.77–1.40) | 0.8187 | − | − |
| Other PD unspecified vs. CCPD | 1.39 (0.97–2.01) | 0.0765 | 1.02(0.70–1.49) | 0.9142 | |||
PD peritoneal dialysis, CCPD continuous cycling peritoneal dialysis, CAPD continuous ambulatory peritoneal dialysis, CAKUT congenital anomalies of the kidney and urinary tract
Adjusted model was controlled by initiation era, age, race, sex, primary diagnosis, and dialysis modality
Final adjusted model was controlled by initiation era, age, race, and sex considering dialysis modality and primary diagnosis were within the causal pathway
Discussion
The decision regarding the initiation of chronic dialysis in infants can be very challenging for families and providers given the medical complexity of the required care and the limited patient outcome data. The challenges have been well documented in surveys of pediatric dialysis care providers, as well as in a recent publication that highlighted ethical considerations associated with infant dialysis [21, 22]. We sought to collect and analyze data from a large US population-based registry of infants diagnosed with end-stage kidney disease to expand upon prior reports pertaining to the outcome of those who received chronic dialysis. This information could help inform the decision making process regarding dialysis initiation for both healthcare providers and affected family members. Most importantly, the non-voluntary nature of the data collected by the USRDS permitted a more complete assessment of patient outcomes compared to prior reports.
The majority of infants on CPD in the USRDS registry were non-Hispanic, white males with CAKUT who were prescribed CCPD. These demographics are very similar to data previously reported by the NAPRTCS for the cohort of infants in their dialysis registry [21, 22].
The median time on dialysis before first transplant was observed to be longer in all infants in this cohort (1.5 to 2.5 years) in comparison to the prior reports. In the USRDS analysis of all ESKD patients < 5 years of age by Mitsnefes et al., the mean dialysis duration prior to initial transplant was 1.3 years; similarly, the NAPRTCS has reported a mean time from dialysis to transplant of 1.4 years for infants on CPD [23]. Our longer reported duration of dialysis is in part related to the larger number of neonates in our cohort who require a longer time on dialysis before reaching the appropriate size for kidney transplantation.
Neonates with ESKD are among the most complex patients to manage on dialysis, although more recent data based on voluntary registry information has reflected improved survival characteristics. Earlier studies found 1-year survival rates of around 50% for patients who initiated CPD as neonates [5, 6]. In contrast, more recent data published by van Stralen et al. on 264 patients who initiated CPD at ≤ 1 month of age demonstrated 2- and 5-year survival rates of 81% and 76%, respectively between 2000 and 2011 [10]. Similarly, Carey et al. reported 3-year survival rates among neonates on CPD of 79% from 2000 to 2012 [14]. We also saw this trend of improved survival over time among neonates in the USRDS cohort. In our analysis, the 1- and 5-year patient survival rates for children who initiated CPD as neonates improved by approximately 10% when comparing the more recent era (2000–2014) to the prior cohort (1990–1999). During the more contemporary era, the 5-year survival for the older infants was nearly 20% greater compared to the earlier era of dialysis initiation. Most importantly, our cumulative incidence competing risk model substantiated the significance of the improved survival in neonates and older infants that has occurred over time. Though not specifically analyzed in this cohort, we speculate that the improved survival trends may be a reflection of improvements in our understanding and clinical application of a variety of factors including nutrition, infection prevention, and dialysis technology [17, 18, 24–26].
Prior studies have reported superior survival among older infants (> 1 month of age) initiated on CPD compared to neonates. The reason for the difference is likely multifactorial, and influential factors might include the technical challenges associated with dialysis initiation in smaller infants, perhaps a greater incidence of comorbidities associated with dialysis initiation at a younger age, and a higher likelihood for the presence of more severe kidney disorders associated with a greater mortality risk (i.e., polycystic kidney disease) which prompted dialysis initiation soon after birth [2, 7, 14]. In an analysis extracted from the ESPN/ERA-EDTA registry by Vidal et al. of 917 infants ≤ 1 year of age at initiation of CPD from 1991 to 2013, younger age at dialysis initiation was, in fact, a significant risk factor for death, with a 5% lower risk of death per month of later initiation [12]. Similarly, in the recent report published by Carey et al. from the NAPRTCS registry, of all 628 infants < 1 year of age on CPD, the 3-year survival was 79% in the more contemporary era (2000–2012) for neonates compared to an approximately 85% 3-year survival in the older infants receiving CPD [14]. We did not see this trend among older infants initiated on dialysis compared to neonates in the USRDS cohort. Our competing risk model demonstrated that age was not an independent risk factor for patient mortality. In part, this may be due to the larger proportion of older infants in the USRDS cohort with polycystic kidney disease, a primary renal disorder recognized to be associated with a substantial mortality risk when accompanied by the need for chronic dialysis during infancy.
The competing risk multivariate model also demonstrated an increased risk for mortality in female infants. This finding of increased mortality among female infants on CPD is unique to our cohort. While the percentage of females were represented equally in the two initiation eras as it pertains to age groups and primary diagnoses, compared to males (63%), fewer females (56%) received a kidney transplant during the entire follow-up period from 1990 to 2014. We speculate that poorer rates of transplantation in female patients are potentially the result of poorer survival outcomes for female infants on CPD. Poorer rates of transplantation in female children on dialysis have been previously reported [27].
Of the 360 (20.9%) infants overall who died while receiving chronic dialysis during the follow-up period, the majority of deaths were attributed to cardiorespiratory compromise, infection, or an unknown cause. In fact, in patients initiated on CPD during the contemporary era, a disproportionately higher number of infants died due to infections, as was seen by VanStralen et al. [10]. These causes of mortality are consistent with data recently published regarding the provision of dialysis in infants from the ESPN/ERA-EDTA registry and emphasize the importance of fluid and blood pressure management, as well as infection prevention efforts like those of the SCOPE collaborative, which have resulted in a decreased rate of peritonitis in children on CPD [12, 28].
Finally, the cumulative cause-specific hazard plot demonstrates that the majority of patient deaths primarily occurred within the first 2 years of dialysis during both eras of dialysis initiation. This finding highlights the complex nature of dialysis in the youngest infants, especially when other comorbidities exist, and the risk of early technical complications like those recently described by the International Pediatric Peritoneal Dialysis Network (IPPN) [6, 10, 12, 14, 29, 30].
Within the 10+-year follow-up period, 61.5% of this entire cohort received at least one kidney transplant. At 5 years post-dialysis initiation, combined data extracted from the ESPN/ERA-EDTA, IPPN, ANZDATA, and Japanese RRT registries demonstrated that 55% of infants had received a kidney transplant [10]. More recently, data published by the ESPN/ERAEDTA in 2017 demonstrated that 70% of infants who initiated dialysis during the first year of life received a kidney transplant within 5 years [12]. The fact that rates of transplantation are not higher in this USRDS cohort over the course of a 10+-year follow-up period likely indicates that the majority of infants on dialysis who undergo kidney transplantation do so within the first 5 years after dialysis initiation.
The strength of this study rests with the fact that we have described the largest and most complete cohort of infants who initiated chronic dialysis at < 1 year of age to date by virtue of the unique, non-voluntary nature of the data collection. There are, however, several limitations to this study. First, although the USRDS database captures information on all patients in the US with ESKD who have transitioned to chronic outpatient dialysis, it is likely that the analysis of patient outcomes are confounded by missing data on infants who initiated CPD for presumed ESKD but died prior to hospital discharge and completion of form 2728 required for entry into the USRDS database. Second, the survival data is also confounded by those neonates/infants with ESKD for whom the provision of chronic dialysis therapy was either withdrawn prior to completion of form 2728 or was refused by the family. Third, by separating patients by dialysis initiation era, several patients who initiated dialysis near the end of the earlier initiation era inevitably received some dialysis during the more contemporary era. These few patients may have contributed to the appearance of better survival data in the earlier initiation era as a result of actually benefiting from advanced dialysis technology and nutritional support in the more recent era. Finally, the USRDS registry was initially designed for the monitoring of adult patients with ESKD; hence, the capacity to draw conclusions from the database is limited by large amounts of missing data (i.e., comorbidities) and/or the absence of data fields pertinent to pediatric patients (i.e., birth weight, gestational age).
Conclusion
In summary, the provision of chronic dialysis during infancy remains uniquely challenging for healthcare providers and affected families alike. However, as a result of advances in medical technology and our better understanding and implementation of important therapeutic interventions, our data and that of others provides evidence of improved survival for even the youngest infants who receive chronic peritoneal dialysis and the chance for successful transplantation. Nevertheless, mortality rates remain high for these complex patients, and additional research should be directed toward a variety of issues (e.g., cardiovascular and neurocognitive outcome, infection prevention, family dynamics) that impact both the quantity and quality of life for these young and very vulnerable group of children.
Acknowledgments
Funding source The primary author is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR001109. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- USRDS
United States Renal Data System
- ESKD
End-stage kidney disease
- NAPRTCS
North American Pediatric Renal Trials and Collaborative Studies
- ANZDATA
Australia and New Zealand Dialysis and Transplant Registry
- ESPN/ERA-EDTA
European Society for Pediatric Nephrology/European Renal Association–European Dialysis and Transplant Association
- KDOQI
Kidney Disease Outcomes Quality Initiative
- IPPN
International Pediatric Peritoneal Dialysis Network
- CPD
Chronic peritoneal dialysis
- RRT
Renal replacement therapy
- IQR
Interquartile range
- HR
Hazard ratio
Footnotes
The study was approved by the Institutional Review Boards of Children’s Mercy Kansas City and the University of North Carolina.
Conflict of interest The authors declare that they have no conflicts of interest.
Financial disclosure The authors have no financial relationships relevant to this article to disclose.
“The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author (s) and in no way should be seen as an official policy or interpretation of the US government.”
References
- 1.System USRD (2017) 2017 annual data report: atlas of pediatric end-stage renal disease in the United States United States renal data system (USRDS). National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; https://www.usrds.org/2017/view/v2_07.aspx [Google Scholar]
- 2.Chavers BM, Molony JT, Solid CA, Rheault MN, Collins AJ (2015) One-year mortality rates in US children with end-stage renal disease. Am J Nephrol 41:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coulthard MG, Crosier J (2002) Outcome of reaching end stage renal failure in children under 2 years of age. Arch Dis Child 87: 511–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(2011) North American Pediatric Renal Trials and Collaborative Studies: NAPRTCS 2011 Annual Dialysis Report. The EMMES Corporation, Rockville, MD: [cited December 17, 2017] https://web.emmes.com/study/ped/annlrept/annualrept2011.pdf [Google Scholar]
- 5.Carey WA, Talley LI, Sehring SA, Jaskula JM, Mathias RS (2007) Outcomes of dialysis initiated during the neonatal period for treatment of end-stage renal disease: a north American pediatric renal trials and collaborative studies special analysis. Pediatrics 119: e468–e473 [DOI] [PubMed] [Google Scholar]
- 6.Rheault MN, Rajpal J, Chavers BM, Nevins TE (2009) Outcomes of infants <28 days old treated with peritoneal dialysis for end-stage renal disease. Pediatr Nephrol 24:2035–2039 [DOI] [PubMed] [Google Scholar]
- 7.Mitsnefes MM, Laskin BL, Dahhou M, Zhang X, Foster BJ (2013) Mortality risk among children initially treated with dialysis for end-stage kidney disease, 1990–2010. JAMA 309:1921–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verrina E, Zacchello G, Perfumo F, Edefonti A, Sorino P, Bassi S, Andreetta B, Cattarelli D, Capasso G, Consalvo G et al. (1995) Clinical experience in the treatment of infants with chronic perito-neal dialysis. Adv Perit Dial 11:281–284 [PubMed] [Google Scholar]
- 9.Chesnaye NC, Bonthuis M, Schaefer F, Groothoff JW, Verrina E, Heaf JG, Jankauskiene A, Lukosiene V, Molchanova EA, Mota C, Peco-Antić A, Ratsch IM, Bjerre A, Roussinov DL, Sukalo A, Topaloglu R, Van Hoeck K, Zagozdzon I, Jager KJ, Van Stralen KJ, ESPN/ERA–EDTA registry (2014) Demographics of paediatric renal replacement therapy in Europe: a report of the ESPN/ERAEDTA registry. Pediatr Nephrol 29:2403–2410 [DOI] [PubMed] [Google Scholar]
- 10.van Stralen KJ, Borzych-Duzalka D, Hataya H, Kennedy SE, Jager KJ, Verrina E, Inward C, Ronnholm K, Vondrak K, Warady BA, Zurowska AM, Schaefer F, Cochat P (2014) Survival and clinical outcomes of children starting renal replacement therapy in the neonatal period. Kidney Int 86:168–174 [DOI] [PubMed] [Google Scholar]
- 11.Laakkonen H, Lonnqvist T, Valanne L, Karikoski J, Holmberg C, Ronnholm K (2011) Neurological development in 21 children on peritoneal dialysis in infancy. Pediatr Nephrol 26:1863–1871 [DOI] [PubMed] [Google Scholar]
- 12.Vidal E, van Stralen KJ, Chesnaye NC, Bonthuis M, Holmberg C, Zurowska A, Trivelli A, Da Silva JEE, Herthelius M, Adams B, Bjerre A, Jankauskiene A, Miteva P, Emirova K, Bayazit AK, Mache CJ, Sanchez-Moreno A, Harambat J, Groothoff JW, Jager KJ, Schaefer F, Verrina E (2017) Infants requiring maintenance dialysis: outcomes of hemodialysis and peritoneal dialysis. Am J Kidney Dis 69:617–625 [DOI] [PubMed] [Google Scholar]
- 13.Pollack S, Eisenstein I, Tarabeih M, Shasha-Lavski H, Magen D, Zelikovic I (2016) Long-term hemodialysis therapy in neonates and infants with end-stage renal disease: a 16-year experience and outcome. Pediatr Nephrol 31:305–313 [DOI] [PubMed] [Google Scholar]
- 14.Carey WA, Martz KL, Warady BA (2015) Outcome of patients initiating chronic peritoneal dialysis during the first year of life. Pediatrics 136:e615–e622 [DOI] [PubMed] [Google Scholar]
- 15.Feinstein S, Rinat C, Becker-Cohen R, Ben-Shalom E, Schwartz SB, Frishberg Y (2008) The outcome of chronic dialysis in infants and toddlers—advantages and drawbacks of haemodialysis. Nephrol Dial Transplant 23:1336–1345 [DOI] [PubMed] [Google Scholar]
- 16.Kovalski Y, Cleper R, Krause I, Davidovits M (2007) Hemodialysis in children weighing less than 15 kg: a single-center experience. Pediatr Nephrol 22:2105–2110 [DOI] [PubMed] [Google Scholar]
- 17.White CT, Gowrishankar M, Feber J, Yiu V (2006) Clinical practice guidelines for pediatric peritoneal dialysis. Pediatr Nephrol 21: 1059–1066 [DOI] [PubMed] [Google Scholar]
- 18.Foundation KDNK (2006) K/DOQI clinical practice recommendations for peritoneal dialysis adequacy. Am J Kidney Dis 48:S130–S158 [DOI] [PubMed] [Google Scholar]
- 19.Fine J, Gray RJ (1999) A proportional hazards model for subdistribution of a competing risk. J Am Stat Assoc 94:496–509 [Google Scholar]
- 20.Noordzij M, Leffondre K, van Stralen KJ, Zoccali C, Dekker FW, Jager KJ (2013) When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant 28:2670–2677 [DOI] [PubMed] [Google Scholar]
- 21.Teh JC, Frieling ML, Sienna JL, Geary DF (2011) Attitudes of caregivers to management of end-stage renal disease in infants. Perit Dial Int 31:459–465 [DOI] [PubMed] [Google Scholar]
- 22.Lantos JD, Warady BA (2013) The evolving ethics of infant dialysis. Pediatr Nephrol 28:1943–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verrina E, Edefonti A, Gianoglio B, Rinaldi S, Sorino P, Zacchello G, Lavoratti G, Maringhini S, Pecoraro C, Calevo MG, Turrini Dertenois L, Perfumo F (2004) A multicenter experience on patient and technique survival in children on chronic dialysis. Pediatr Nephrol 19:82–90 [DOI] [PubMed] [Google Scholar]
- 24.Warady BA, Bakkaloglu S, Newland J, Cantwell M, Verrina E, Neu A, Chadha V, Yap HK, Schaefer F (2012) Consensus guidelines for the prevention and treatment of catheter-related infections and peritonitis in pediatric patients receiving peritoneal dialysis: 2012 update. Perit Dial Int 32(Suppl 2):S32–S86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warady BA, Neu AM, Schaefer F (2014) Optimal care of the infant, child, and adolescent on dialysis: 2014 update. Am J Kidney Dis 64:128–142 [DOI] [PubMed] [Google Scholar]
- 26.Warady BA, Schaefer F, Alexander SR (2012) Pediatric Dialysis. Springer, New York [Google Scholar]
- 27.Hogan J, Couchoud C, Bonthuis M, Groothoff JW, Jager KJ, Schaefer F, Van Stralen KJ (2016) Gender disparities in access to pediatric renal transplantation in Europe: data from the ESPN/ERA-EDTA registry. Am J Transplant 16:2097–2105 [DOI] [PubMed] [Google Scholar]
- 28.Neu AM, Richardson T, Lawlor J, Stuart J, Newland J, McAfee N, Warady BA (2016) Implementation of standardized follow-up care significantly reduces peritonitis in children on chronic peritoneal dialysis. Kidney Int 89:1346–1354 [DOI] [PubMed] [Google Scholar]
- 29.Hijazi R, Abitbol CL, Chandar J, Seeherunvong W, Freundlich M, Zilleruelo G (2009) Twenty-five years of infant dialysis: a single center experience. J Pediatr 155:111–117 [DOI] [PubMed] [Google Scholar]
- 30.Borzych-Duzalka D, Aki TF, Azocar M, White C, Harvey E, Mir S, Adragna M, Serdaroglu E, Sinha R, Samaille C, Vanegas JJ, Kari J, Barbosa L, Bagga A, Galanti M, Yavascan O, Leozappa G, Szczepanska M, Vondrak K, Tse KC, Schaefer F, Warady BA (2017) Peritoneal dialysis access revision in children: causes, interventions, and outcomes. Clin J Am Soc Nephrol 12:105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]