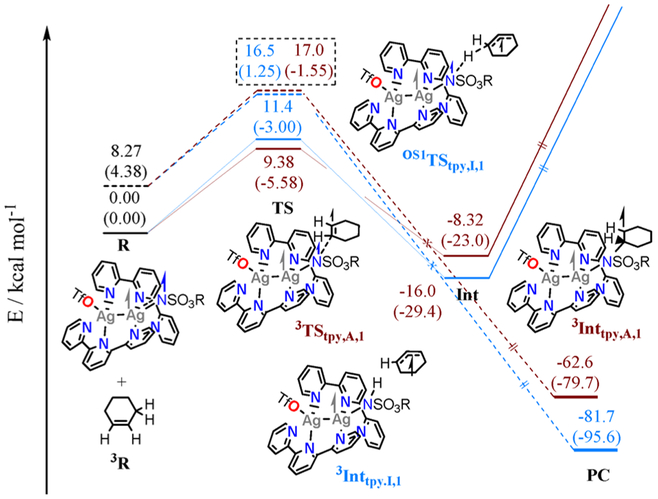
Figure 3.
Reaction coordinate of nitrene transfer from A′(N) to 1 to give either C‒H insertion (blue) or aziridine products (brown). R = reactant, TS = transition state, Int = intermediate, and PC = product complex. Subscript I = C‒H insertion and A = aziridination and superscript OS1 and 3 denote open-shell singlet and triplet spin states, respectively. Solid lines denote the triplet PES, and dashed lines denote the open-shell singlet PES. Arrows and the size of the arrows represent α or β spin populations for the electrons on Ag (silver), N (blue), and organic substrate (black). Black arrows inside the ring represent electron density delocalized over the allylic radical, and arrows outside the ring represent electron density localized on the carbon proximal to the arrow. Numbers shown are Gibbs free energies, and numbers enclosed in parentheses are enthalpies, both taking into account the solvent (CH2Cl2).