Table 4.
Tunable Amination of C=C and C‒H Bonds*
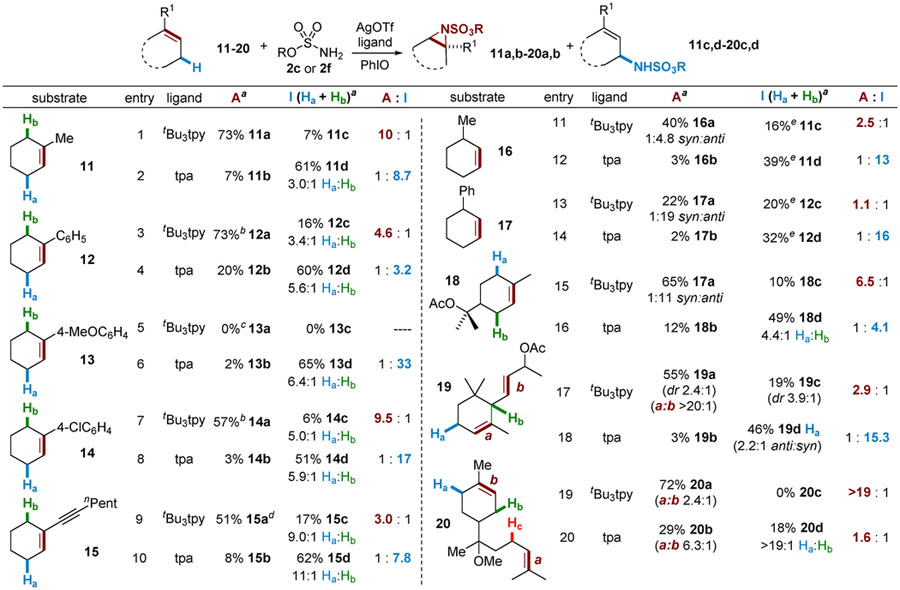
|
A: 10 mol % of AgOTf, 12 mol % of tBu3tpy, HfsNH2 (0.25 mmol), 3.5 equiv of PhIO, CH2Cl2, 4 Å MS, 4 h, rt. I: 10 mol % of AgOTf, 12 mol % of tpa, DfsNH2 (0.25 mmol). 1.2 equiv of PhIO, CH2Cl2 4 Å MS, 1 h, rt.
Isolated yields.
Aziridine was opened with MeOH.
Formation of the epoxide as the major product.
56% NMR yield of aziridine, isolated as the aldehyde in 51% yield.
Transposition of the allylic bond was noted to give the trisubstituted alkene as the product. The ratio of expected to allylic product was approximately 1:1, while transposition was highly dominant for 17.
