Abstract
Sendai virus (SeV) is an enveloped, single-stranded RNA virus of the family Paramyxoviridae. SeV is a useful tool to study its infectious pathomechanism in immunology and the pathomechanism of a murine model of IgA nephropathy. Virus quantification is essential not only to determine the original viral titers for an appropriate application, but also to measure the viral titers in samples from the harvests from experiments. There are mainly a couple of units/titers for Sendai viral quantification: plaque-forming units (PFU) and hemagglutination (HA) titer. Of these, we here describe a protocol for Sendai virus plaque assay to provide PFU using LLC-MK2 cells (a rhesus monkey kidney cell lines) and Guinea pig red blood cells. This traditional protocol enables us to determine Sendai virus PFU in viral stock as well as samples from your experiments.
Keywords: Sendai virus, Plaque assay, Titration, Paramyxivirus, Plaque-forming units, PFU
Background
SeV is a mouse parainfluenza virus type I (Faisca and Desmecht, 2007) that was discovered in Sendai, Japan, in the 1950s (Ishida and Homma, 1978). SeV is a useful tool to study its infection and immune reaction ( Fensterl et al., 2008 ; Chattopadhyay et al., 2010 , 2011 and 2013; Yamashita et al., 2012a , 2012b and 2013; Veleeparambil et al., 2018 ) and the pathomechanism of a SeV-induced of IgA nephropathy ( Yamashita et al., 2007 ; Chintalacharuvu et al., 2008 ). SeV is Precise viral quantification is essential to perform animal and cell culture experiments using an appropriate dose of SeV and also to obtain correct results from experimental samples containing SeV. In 1970s, SeV was quantitated by inoculation into embryonated eggs using hemagglutinin production as a criterion for infection ( Shibuta et al., 1971 ). This method is highly sensitive but time consuming and complex. Therefore, a kidney cell-based plaque assay, a simple and reliable assay using hemadsorption (the attachment of red blood cells to the surface of cell monolayers infected with virus) has been developed (Jessen et al., 1987). This protocol provides a method for SeV PFU using LLC-MK2 cells (a rhesus monkey kidney cell lines) and Guinea pig red blood cells. This method can be applied for most types of samples including cell culture media, cell lysates, tissue homogenates, serum, urine, and bronchoalveolar lavage.
Materials and Reagents
96-well Polypropylene 1.2 ml Cluster Tubes (Sigma-Aldrich, catalog number: CLS4401-960EA)
CorningTM 6-well plate (Thermo Fisher Scientific, catalog number: 07-200-83)
CorningTM 10 ml pipettes (Thermo Fisher Scientific, catalog number: 07-200-574)
15 ml tubes (Thermo Fisher Scientific, catalog number: 12-565-268)
Sendai virus (ATCC, catalog number: ATCC VR-105) as positive control
HyClone® Characterized Fetal Bovine Serum, U.S. Origin (GE Healthcare Life Sciences, catalog number: SH30071.03HI)
GibcoTM Gentamicin, 10 mg/ml (Thermo Fisher Scientific, catalog number: 11500506)
L-Glutamine, 200 mM (Thermo Fisher Scientific, catalog number: A2916801)
-
LLC-MK2 Original (ATCC, catalog number: CCL-7)
Note: These cells are maintained in Medium 199 (Thermo Fisher Scientific, catalog number: 11150-067) containing 5% Fetal Bovine Serum, 20 μg/ml gentamicin, and 2 mM L-glutamine (complete Medium 199). LLC-MK2 cells should be used only up to about passage of 50. The plaques will become gradually smaller as the cell line ages.
-
Guinea Pig Blood in Alsevers (Rockland antibodies & assays, catalog number: R305-0050)
Note: This needs to be less than 2 weeks old.
Medium 199 (10x) (Thermo Fisher Scientific, catalog number: 11825015)
Medium 199 (1x) (Thermo Fisher Scientific, catalog number: 11150067)
HBSS, calcium, magnesium (Thermo Fisher Scientific, catalog number: 14025092)
Sodium Bicarbonate 7.5% solution (Thermo Fisher Scientific, catalog number: 25080094)
BD Difco Agar (Fisher Scientific, catalog number: DF0812-17-9)
-
Trypsin (0.25%), phenol red (Thermo Fisher Scientific, catalog number: 15050065)
Note: The final concentration is 0.00025% (2.5 μg/ml). This reagent needs optimization for a particular lot. 2.5 μg/ml ± 0.25 μg/ml can make the difference between nice large plaques and the cells detached from the plates.
Sterile PBS (with Ca2+ and Mg2+)
Penicillin-Streptomycin (5,000 U/ml) (Thermo Fisher Scientific, catalog number: 15070063)
Complete 2x medium (see Recipes)
Equipment
Multichannel pipette (Gilson, catalog number: FA10015)
Pipettes, P20, P200, P1000 (Gilson, catalog number: F167300)
Biosafety cabinet (Thermo Fisher Scientific, catalog number: 1305)
Tissue culture incubator (at 37 °C with 5% CO2) (Thermo Fisher Scientific, catalog number: 51025983)
100 ml glass bottles (Research Products International, catalog number: 219510)
PrecisionTM General Purpose Water bath (Thermo Fisher Scientific, catalog number: TSGP02)
Autoclave (Steris Amsco Eagle, catalog number: 3021-C Gravity Steam Sterilizer)
Sonic water bath (Skymen Cleaning Equipment, model: JP-008)
Vortex mixer (Research Products International, catalog number: 155560)
Portable Mini Light Box, Benchtop Light Source (Research Products International, catalog number: 815500)
Procedure
Days -5 to -2
Seed LLC-MK2 cells into the necessary number of 6-well plates (as below) to test samples and controls at all needed dilutions.
Note: You want the plates with lightly confluent cells on Day 0 (Figure 1).
Figure 1. Lightly confluent LLC-MK2 cells.
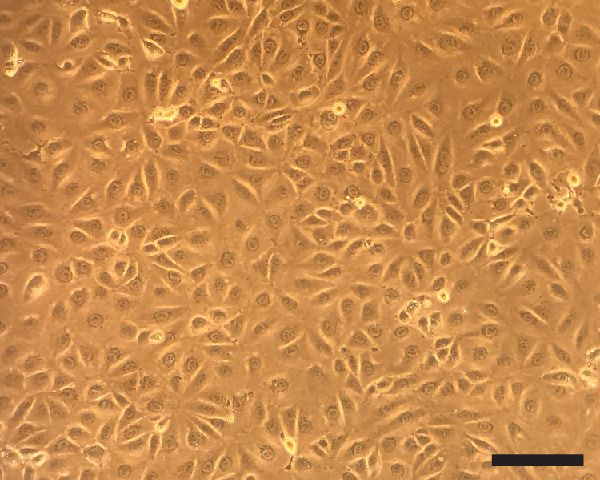
Once overlaid agar is solidified, cells can no longer proliferate. Therefore, the cells want to be confluent when you overlay agar on the cells (Scale bar = 100 μm).
Day 0
-
Set up sterile 50 ml agar solution in 100 ml glass bottle(s) to be used in Step 3f
Add 0.5 g agar to 50 ml distilled water in a 100 ml bottle. One to 4 bottles are needed.
Autoclave the bottle(s) at 121 °C for 30 min.
Mix the agar well when it comes out of the autoclave and hold in a 50 °C water bath until ready to use (Step 3f).
Note: The final 100 ml will handle 8 six-well plates most comfortably at the same time. Up to total 4 bottles and 32 plates are practical.
-
Viral preparation
-
Thaw the virus samples and a positive control (to be titrated) on ice, and keep them on ice.
Note: For the proper analysis, a positive control (Sendai virus, VR-105 from ATCC) is required for every experiment. One vial (1 ml) from ATCC can be used for 10 to 20 experiments. The vial from ATCC should be aliquoted into 50 μl to 100 μl each (10 to 20 tubes) in the first experiment, and stored in liquid nitrogen tank. The viral titer is stable at least for three years by this method according to our experience. We recommend you use the original SeV from ATCC (not a "homemade" SeV stock) as a positive control because of the certainty of the viral titer.
Sonicate (40 kHz frequency and 35 W ultrasonic power) the virus samples for 30 sec in a sonic water bath at room temperature before use to break and dissociate viral chunks.
-
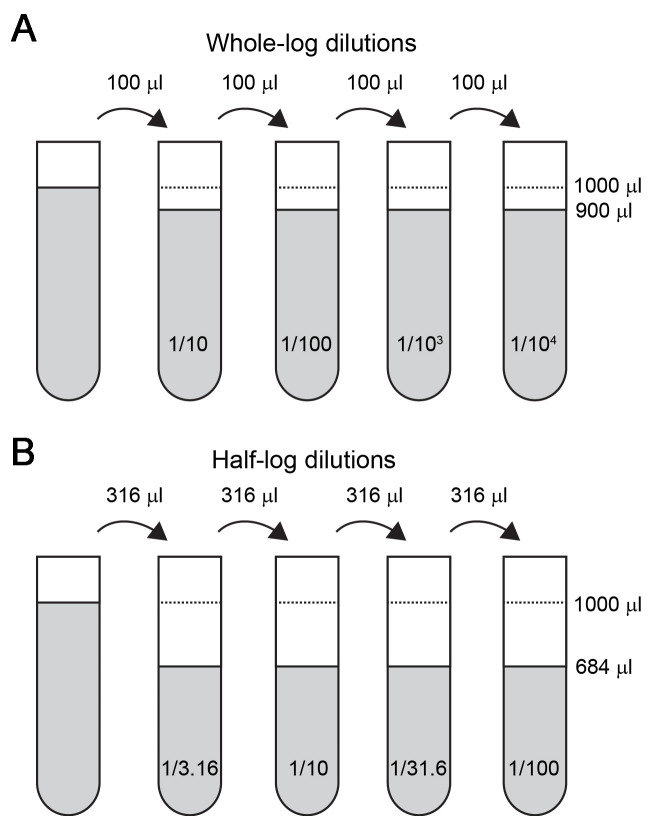
Make serial dilutions of virus in pure Medium 199 (without Fetal Bovine Serum, gentamicin or L-glutamine) at whole log (10-fold) dilutions or half-log (3.16-fold) dilutions in the appropriate range (Figure 2). Doing these dilutions in rack of 96 microtubes (1.2 ml volume) with a multichannel pipette will greatly increase efficiency. Keep samples and dilutions on ice.
Note: "Pure Medium 199" means only Medium 199 (Thermo Fisher Scientific) without serum.
-
-
Inoculation and incubation
Rinse lightly the confluent monolayers of LLC-MK2 cells on the plates with HBSS with Ca2+Mg2+: Remove old media and add 2 ml of HBSS with Ca2+Mg2+.
Suction the media off the number of plates, and quickly add 200 μl of pure Medium 199 to each well.
Add 200 μl of sample dilutions in duplicate or triplicate. Work from the most dilute to the least dilute to save on tip changes.
-
Incubate plates for 90 min at 37 °C with 5% CO2.
Note: Gently agitate plates about every 30 min to avoid drying out the plates.
During the incubation, make up complete 2x media in 50 ml aliquots (see Recipes).
At the end of the 90-min incubation, mix together the complete 2x media (at room temperature) and the 1% agar water (in a 50 °C water bath) for the first set of 8 plates, and place the mixture at room temperature.
Suction viral inoculum media from the plates, and quickly add 2 ml of media with 0.5% agar (Step 3f) into each well using a 10 ml pipette (capacity of 12 ml).
Cool at room temperature until the agar media solidify. Do not stack the plates.
Incubate the plates (cells overlaid with the agar media) for 3 days at 37 °C with 5% CO2.
Figure 2. Serial dilutions of viral sample.
A. For whole-log dilutions, 100 μl of sample will be serially transferred to the next tube that contains 900 μl of pure Medium 199. B. For half-log dilutions, 316 μl of sample will be serially transferred to the next tube that contains 684 μl of pure Medium 199.
Day 3: Hemadsorption
Wash approximately 3 ml of guinea pig blood with sterile PBS (with Ca2+ and Mg2+) 3 times in a 15 ml tube. Spin at 434 × g for 5 min to obtain about 1 ml pellet of red blood cells (RBCs).
Resuspend the 1 ml of RBC pellet to 9 ml of PBS (10% volume/volume) with PBS (with Ca2+ and Mg2+).
Make a 0.1% RBC suspension with PBS (with Ca2+ and Mg2+) from 10% RBC suspension.
Remove 6-well plates from the incubator in small groups (like 4 plates).
-
Shake the agar off one plate at a time with a quick snap of the wrist into a biohazard bag and immediately add 2 ml of PBS (with Ca2+ and Mg2+; at room temperature).
Note: Removal of agar leaves the plate very dry and the cells are vulnerable (LLC-MK2 cells are not particularly sensitive to detachment, but this is an effect of this assay). Please add PBS immediately but gently along the wall of the wells, not directly to the cells.
Remove PBS from the 6-well plates, and add 2 ml of 0.1% RBC solution to each well (Step 3).
Incubate plates at room temperature for 20-30 min.
-
Gently agitate plates to resuspend RBCs, and replaced the RBC suspension with 2 ml PBS (with Ca2+ and Mg2+).
Note: This one-time wash is usually sufficient, but a second wash is acceptable.
-
Score plaques as well-defined areas where RBCs attach (Figure 3).
Note: Clean the bottom (outside) of the well with alcohol before counting. If you place the plates on a light box, you will be able to more easily identify the plaques.
-
Calculate pfu/ml based on sample size (200 μl) and dilution.
An example of titer calculation
If the inoculation sample in Figure 3 is 107 dilution:
Virus titer (pfu/ml) = 5 (plaques)/[0.2 ml (inoculation volume) x 107 (sample dilution)]
= 2.5 x 108 PFU/ml
Figure 3. Sendai virus plaques.
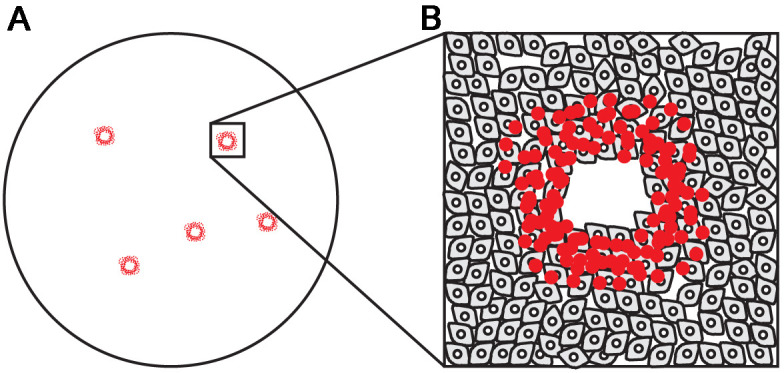
A. Five plaques in one well. B. Magnification of one plaque shows a ring-shape plaque of red blood cells attaching to LLC-MK2 cells and a central empty area where LLC-MK2 cells died and detached.
Notes
This protocol needs an appropriate facility (Biosafety level 2) and an approved Institutional Biosafety Committee (IBC) protocol.
Recipes
-
Complete 2x medium
Note: All components need to be sterile before mixing.
Volume (ml unless otherwise indicated) Final total volume 20 25 30 40 50 70 Sterile H2O 14.38 17.98 21.58 28.77 35.97 50.35 10x Medium 199 4 5 6 8 10 14 Penicillin-Streptomycin 0.4 0.5 0.6 0.8 1.0 1.4 Sodium Bicarbonate,7.5% solution1.18 1.47 1.76 2.35 2.93 4.11 Trypsin, 0.25% 40 μl 50 μl 60 μl 80 μl 100 μl 140 μl
Acknowledgments
Dr. Yamashita is supported by American Heart Association Scientist Development Grant (17SDG33660947) and University of California Los Angeles Clinical and Translational Science Institute Grant (ΜL1TR001881). We would like to thank Dr. Steven Emancipator for the mentoring in the early stage of Dr. Yamashita's career development and Dr. John Nedrud for previous collaboration.
Competing interests
The authors do not have any potential conflicts of interest or competing interests to declare.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Chattopadhyay S., Fensterl V., Zhang Y., Veleeparambil M., Yamashita M. and Sen G. C.(2013). Role of interferon regulatory factor 3-mediated apoptosis in the establishment and maintenance of persistent infection by Sendai virus. J Virol 87(1): 16-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chattopadhyay S., Marques J. T., Yamashita M., Peters K. L., Smith K., Desai A., Williams B. R. and Sen G. C.(2010). Viral apoptosis is induced by IRF-3-mediated activation of Bax. EMBO J 29(10): 1762-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chattopadhyay S., Yamashita M., Zhang Y. and Sen G. C.(2011). The IRF-3/Bax-mediated apoptotic pathway, activated by viral cytoplasmic RNA and DNA, inhibits virus replication. J Virol 85(8): 3708-3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chintalacharuvu S. R., Yamashita M., Bagheri N., Blanchard T. G., Nedrud J. G., Lamm M. E., Tomino Y. and Emancipator S. N.(2008). T cell cytokine polarity as a determinant of immunoglobulin A(IgA) glycosylation and the severity of experimental IgA nephropathy. Clin Exp Immunol 153(3): 456-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Faisca P. and Desmecht D.(2007). Sendai virus, the mouse parainfluenza type 1: a longstanding pathogen that remains up-to-date. Res Vet Sci 82(1): 115-125. [DOI] [PubMed] [Google Scholar]
- 6. Fensterl V., White C. L., Yamashita M. and Sen G. C.(2008). Novel characteristics of the function and induction of murine p56 family proteins. J Virol 82(22): 11045-11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ishida N. and Homma M.(1978). Sendai virus. Adv Virus Res 23: 349-383. [DOI] [PubMed] [Google Scholar]
- 8. Jessen R. H., Nedrud J. G. and Emancipator S. N.(1987). A mouse model of IgA nephropathy induced by Sendai virus. Adv Exp Med Biol 216B: 1609-1618. [PubMed] [Google Scholar]
- 9. Shibuta H., Akami M. and Matumoto M.(1971). Plaque formation by sendai virus of parainfluenza virus group, type 1 on monkey, calf kidney and chick embryo cell monolayers. Jpn J Microbiol 15(2): 175-183. [DOI] [PubMed] [Google Scholar]
- 10. Veleeparambil M., Poddar D., Abdulkhalek S., Kessler P. M., Yamashita M., Chattopadhyay S. and Sen G. C.(2018). Constitutively bound EGFR-mediated tyrosine phosphorylation of TLR9 is required for its ability to signal. J Immunol 200(8): 2809-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yamashita M., Chattopadhyay S., Fensterl V., Saikia P., Wetzel J. L. and Sen G. C.(2012). Epidermal growth factor receptor is essential for Toll-like receptor 3 signaling. Sci Signal 5(233): ra50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamashita M., Chattopadhyay S., Fensterl V., Zhang Y. and Sen G. C.(2012). A TRIF-independent branch of TLR3 signaling. J Immunol 188(6): 2825-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamashita M., Chintalacharuvu S. R., Kobayashi N., Nedrud J. G., Lamm M. E., Tomino Y. and Emancipator S. N.(2007). Analysis of innate immune responses in a model of IgA nephropathy induced by Sendai virus. Contrib Nephrol 157: 159-163. [DOI] [PubMed] [Google Scholar]
- 14. Yamashita M., Millward C. A., Inoshita H., Saikia P., Chattopadhyay S., Sen G. C. and Emancipator S. N.(2013). Antiviral innate immunity disturbs podocyte cell function. J Innate Immun 5(3): 231-241. [DOI] [PMC free article] [PubMed] [Google Scholar]