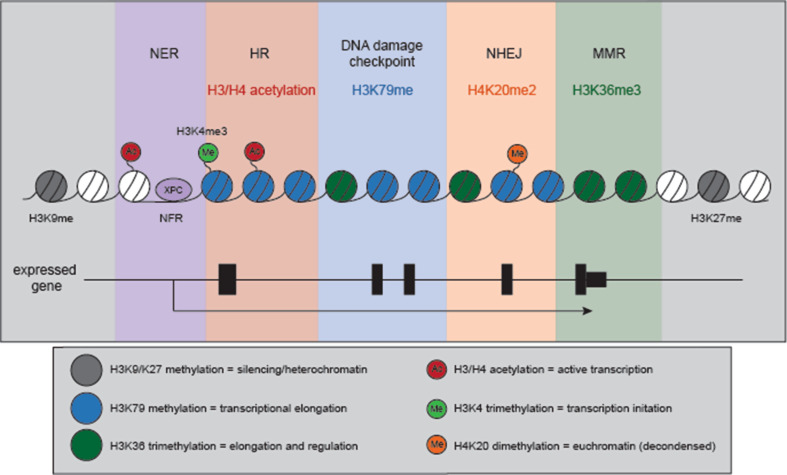
Fig. 3.
Histone modifications associated with both transcription and DNA replication/repair processes. A variety of epigenetic modifications link transcription with DNA replication and repair processes. A subset of proteins and histone modifications appear to play dual roles, and may allow for crosstalk in S phase. The promoters of active genes predominantly contain a nucleosome free region (NFR) 5′ of the transcription start site (TSS). Interestingly, the DNA repair proteins involved in transcription-coupled repair XPC, XPG and XPF have been found to play a role in regulating transcriptional activity at a subset of genes, and occupy promoters at the NFR, like many transcription factors (purple panel). Acetylation of histones H3 and H4 promotes transcriptional activity by enhancing chromatin accessibility and the adoption of a euchromatic state. H3/H4 acetylation also increases with homologous recombination (HR). Similar to its role in transcription, H3/H4 acetylation makes the DNA surrounding the DSB “accessible” for repair. HR requires the invasion of a homologous template (either the sister chromatid or homologous chromosome) to prime new DNA synthesis and repair the damaged region. Thus, “open” chromatin surrounding the DNA break and the repair template would increase repair efficiency (red panel). H3 methylation on lysine 79 (H3K79me1/2) is important for telomeric silencing and heterochromatin maintenance, but also promotes transcriptional elongation. It also correlates with replication initiation regions, and depletion of the sole H3K79 methyltransferase DOT1L can induce over-replication and genome instability (blue panel). Lysine 20 dimethylation of H4 (H4K20me2) is a common mark of euchromatin, and is bound by the Tudor domain of the DNA repair regulator 53BP1. 53BP1 binding promotes NHEJ by limiting DNA resection, suppressing RPA association and the recruitment of HR proteins (orange panel). H3 lysine 36 trimethylation (H3K36me3) increases along a gene body, peaking in the 3′end of a transcribed region. Recent work has uncovered a role for H3K36me3 in regulating DNA mismatch repair (MMR), recruiting MutSα (Msh2-Msh6) through direct interaction with the Msh6 subunit even in the absence of DNA mismatches ([109], green panel). In the diagram of a gene body in the lower panel, black boxes mark exons while the thin black line represents non-coding DNA