Figure 1. Schwann cell EED is required for timely axon regeneration.
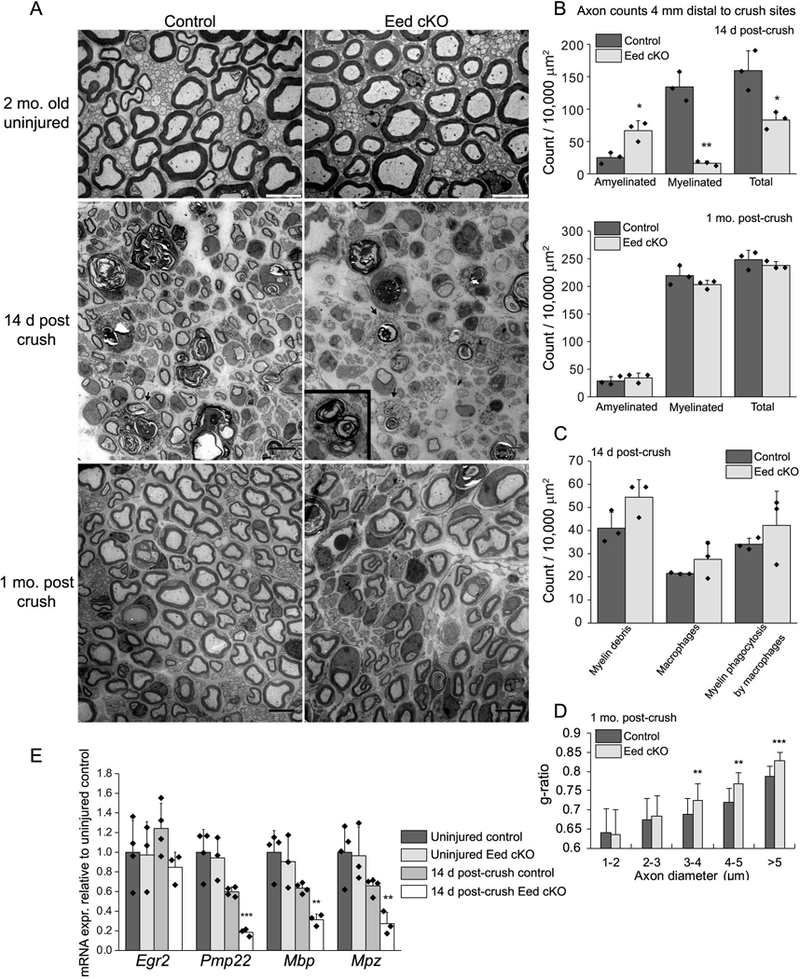
A, Electron micrographs of the sciatic nerves at the indicated time points after crush and uninjured nerves of Eed cKO mice and littermate controls. The arrows and inset show macrophages engulfing myelin debris. Scale bars of uninjured and injured nerve images are 5 μm and 8 μm, respectively. B, C, Myelinated and amyelinated axons (> 1μm in diameter), myelin debris, macrophages, and engulfed myelin by macrophages were counted in randomly selected fields that accounted for over 40% of an entire sciatic nerve cross section from each animal and normalized per surface area (10,000 μm2). Data: mean ± STDEV; **p < 0.005, *p < 0.05; n=3 per genotype (one-way ANOVA). D, For g-ratio analysis (axon diameter/diameter of myelinated fiber), the diameter of axon and outer diameter of myelinated fiber were measured on over 500 randomly selected fibers per genotype. Data: weighted mean ± pooled STDEV; ***p < 0.0005, **p < 0.005, *p < 0.05; n=3 per genotype and age (one-way ANOVA). E, qRT-PCR analysis was used to identify the expression level of myelin genes from 2 month Eed cKO and control sciatic nerves in uninjured condition or 14 day after crush. Expression levels were normalized with Gapdh. Asterisks indicate p-value between genotypes in the respective condition. Data: mean ± SD; **p < 0.005, ***p < 0.0005; n=4 for control and n=3 for Eed cKO (one-way ANOVA).
