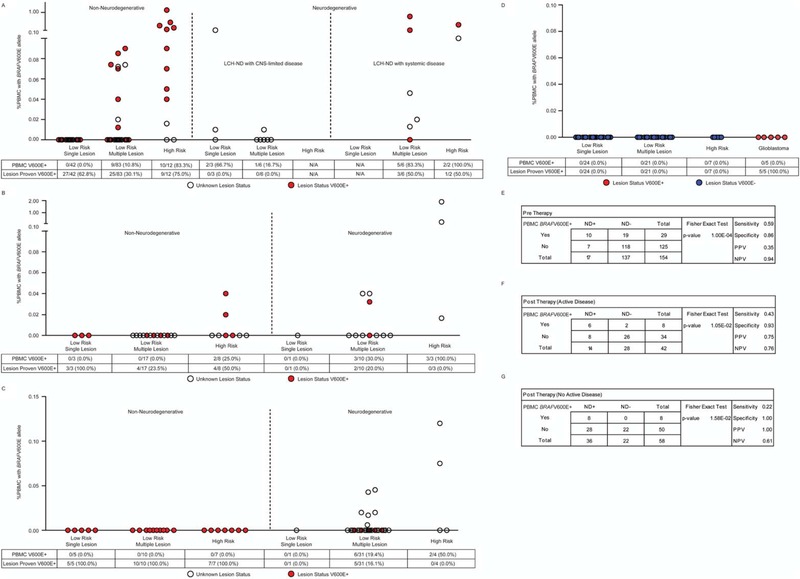
Figure 4.
Identification of cells with BRAFV600E in peripheral blood of patients with non-neurodegenerative and Langerhans cell histiocytosis with neurodegenerative disease (LCH-ND) at specific time points. (A) BRAFV600E DNA was evaluated using quantitative polymerase chain reaction in peripheral blood mononuclear cells (PBMCs) in pre-chemotherapy non-neurodegenerative patients with active disease outside the central nervous system (CNS) (left), LCH-ND patients with no active disease outside the CNS (middle), and LCH-ND patients with active disease outside the CNS (right). PBMCs were evaluated for BRAFV600E in post-chemotherapy patients who were treated previously with systemic therapy at (B) relapse with active disease outside the CNS in non-neurodegenerative (left) and LCH-ND patients (right) and (C) in patients presumed to be cured with no active disease outside the CNS in both non-neurodegenerative (left) and LCH-ND (right) patients. (D) As controls, no BRAFV600E cells were detected in pre-chemotherapy PBMCs from LCH patients with known BRAF wild-type lesions (left) or in PBMCs from 5 pre-therapy pediatric glioblastoma patients with known BRAFV600E+ tumors (right). In panels A-D, the percentage of patients with BRAFV600E detected in PBMCs and the percentage of patients with known BRAFV600E lesion status are indicated below each graph. (E-G) Sensitivity, specificity, and positive and negative predictive values of BRAFV600E in PBMCs for LCH-ND are indicated. Paired analysis (P value) indicates statistical significance of BRAFV600E+ PBMCs between non-ND and LCH-ND groups.