Abstract
Objectives
Previously we demonstrated the benefit of isavuconazole in treating murine mucormycosis due to Rhizopus. We wanted to determine the efficacy of isavuconazole in treating murine mucormycosis caused by Mucor, the second most common cause of the disease. Furthermore, because we previously determined that Rhizopus possesses the target enzyme for echinocandins and micafungin has activity against murine mucormycosis, we compared the activity of combination therapy (isavuconazole + micafungin) with placebo, either drug alone or standard therapy of liposomal amphotericin B (LAmB) in treating pulmonary murine mucormycosis caused by Rhizopus delemar.
Methods
In vitro susceptibility to isavuconazole of Mucorales was evaluated using the CLSI M38-A2 method. Immunosuppressed mice were intratracheally infected with either Mucor circinelloides or R. delemar. Treatment with isavuconazole (orally), micafungin (intraperitoneally), a combination of both or LAmB (intravenously) was compared, with survival and tissue fungal burden serving as primary and secondary endpoints, respectively.
Results
Isavuconazole was as effective as LAmB in prolonging survival of mice infected with M. circinelloides. Against R. delemar-induced mucormycosis, all monotherapy treatments significantly improved survival of mice versus placebo without showing superiority over one another. However, LAmB was superior in lowering fungal burden in target organs. Although combination therapy of isavuconazole + micafungin did not enhance survival of mice over monotherapy, antagonism was not detected between the two drugs.
Conclusion
Isavuconazole is effective in treating pulmonary murine mucormycosis due to Mucor. In addition, combination therapy of isavuconazole + micafungin does not demonstrate synergy and it is not antagonistic against Rhizopus-induced mucormycosis.
Introduction
Mucormycosis is a life-threatening infection that occurs predominantly in patients immunocompromised by diabetic ketoacidosis or neutropenia. Unfortunately, despite current therapy, the overall mortality of mucormycosis remains >50%,1 and it approaches 100% in patients with disseminated disease.2
Mucormycosis is caused by a variety of organisms belonging to the order Mucorales, among which Rhizopus species are the most common cause of infection followed by Mucor species.3 Isavuconazole has an in vitro activity against Mucorales,4,5 and is effective in prolonging the survival of mice infected with Rhizopus delemar.6 The efficacious animal data against R. delemar combined with a recently conducted single-arm open-label clinical trial in treating mucormycosis patients with isavuconazole,7 as well as case–control analysis, resulted in the US FDA approving isavuconazole for treating invasive mucormycosis. However, in vivo data on isavuconazole activity against mucormycosis caused by Mucor species are lacking. Therefore, we wanted to investigate the potential role of isavuconazole in managing mucormycosis due to Mucor.
Despite the lack of in vitro activity of echinocandins against Mucorales, this group of fungi contains the target enzyme for echinocandins.8 In fact, combination of echinocandins with lipid formulation amphotericin B demonstrates synergy in treating murine mucormycosis.9 Additionally, a small retrospective study of patients with rhino-orbital mucormycosis showed enhancement of survival rates in patients treated with caspofungin acetate + lipid formulation amphotericin B over patients treated with the polyene alone.10 Hence, we also investigated the role of combination therapy of isavuconazole + micafungin, in treating mucormycosis.
Methods
Mucorales and culture conditions
R. delemar 99-880 and clinical isolates of Mucor circinelloides f. circinelloides and M. circinelloides f. jenssenii were obtained from the Fungus Testing Laboratory at the University of Texas Health Sciences Center at San Antonio (UTHSCSA). The organisms were grown on potato dextrose agar for 4–7 days at 37°C. The sporangiospores were collected in endotoxin-free PBS containing 0.01% Tween 80, washed with PBS and then counted with a haemocytometer to prepare the final concentration.
Susceptibility testing, immunosuppression and pharmacokinetic studies
The MIC with 100% growth inhibition for isavuconazole (Astellas Pharma US, Inc., Northbrook, IL, USA) was determined against six clinical isolates of M. circinelloides using the CLSI M38-A2 method.11 We previously determined the MIC of isavuconazole for R. delemar or Rhizopus oryzae using the same method.6
Next, the pharmacokinetics of isavuconazole was evaluated in the neutropenic mouse model of intratracheal infection.12 Male CD-1 mice (20–25 g; Envigo, Indianapolis, IN, USA) were used. They were given irradiated feed and sterile water containing 50 mg/L Baytril (enrofloxacin; Bayer, Leverkusen, Germany) ad libitum on day −3, then switched to daily ceftazidime (5 mg/mouse) subcutaneous treatment starting day 0 through day +8 relative to infecting with R. delemar or through day +13 relative to infection with Mucor. Neutropenia was induced by cyclophosphamide [200 mg/kg intraperitonally (ip)] and cortisone acetate (500 mg/kg subcutaneously) on day −2 and +3 relative to infection with R. delemar and with an additional treatment on day +8 when mice were infected with M. circinelloides f. jenssenii. The treatment regimens resulted in ∼10 days (for the two doses regimen) and 15 days (for the three doses regimen) of leucopenia with total white blood cell count dropping from ∼130 000/cm3 to almost no detectable leucocytes as determined by the Unopette System (Becton, Dickinson and Co.). Pharmacokinetic studies were conducted using doses of 80, 110, 215 and 400 mg/kg of the prodrug isavuconazonium sulphate given orally three times a day, for 4 days consecutively. Four hours following the last dose, mice were sacrificed and total blood was collected by cardiac puncture. An analytical method that uses UPLC/MS for the determination of itraconazole and hydroxyl-itraconazole was adapted and validated for the measurement of isavuconazole concentrations.13 Briefly, a standard curve was prepared by spiking blank human plasma with isavuconazole (BAL4815), and the internal standard valethamate bromide (Sigma–Aldrich, St Louis, MO, USA) was added to each sample. All samples were buffered and loaded on to conditioned solid phase extraction columns, which were then washed with independent washings of 5.0% NH4OH and 15.0% methanolic water. After elution of the samples with 1.0 mL of methanol and 1.0 mL of an acidic methanolic mixture (2% formic acid in methanol), the combined eluates were dried under a stream of nitrogen. The dried residues were then reconstituted with 60:40 acetonitrile/water, injected and analysed under specified conditions using a mass to charge ratio (m/z) for isavuconazole of 438.2. The lowest limit of quantification was 0.25 μg/mL isavuconazole.
Infection and treatment
Neutropenic mice were intratracheally infected with 2.5 × 105 spores in 25 μL PBS of M. circinelloides f. jenssenii or R. delemar 99-880 using a gel-loading tip after sedation with isoflurane gas.12 Following inoculation, three mice were sacrificed and their lungs harvested for quantifying the delivered fungal inoculum by quantitative culturing on potato dextrose agar +0.1% triton X-100 (Sigma–Aldrich). Treatment with isavuconazonium sulphate (110 or 215 mg/kg three times daily given orally), micafungin (Astellas Pharma US, Inc.) (1 mg/kg once daily, given ip)9 or combination of isavuconazonium sulphate + micafungin started 16 h post-infection and continued through day +4. Mice treated with a high dose of liposomal amphotericin B (LAmB) [15 mg/kg once daily, given intravenously (iv); Gilead Sciences Inc., Foster City, CA, USA] was included as a positive control.14 The primary and secondary endpoints were time to moribundity of infected mice and tissue fungal burden in lungs and brains (primary and secondary target organs) determined using quantitative PCR, respectively.8
Ethics
Animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, according to the NIH guidelines for animal housing and care. Approval reference number 21450-01.
Statistical analysis
The non-parametric log-rank test was used to determine differences in survival times. Differences in tissue fungal burdens were compared by the non-parametric Wilcoxon rank sum test for multiple comparisons. P < 0.05 was considered significant.
Results and discussion
Isavuconazole is active against M. circinelloides in vitro and in vivo
We determined the in vitro activity of isavuconazole against three clinical strains of M. circinelloides f. jenssenii (UTHSCSA DI15-129; DI15-130; DI15-131) and three strains of M. circinelloides f. circinelloides (UTHSCSA DI15-126; DI15-127; DI15-128). Isavuconazole had an MIC of 4 mg/L for all three strains of M. circinelloides f. jenssenii. Slightly elevated MICs were detected with M. circinelloides f. circinelloides with 8 mg/L for strains DI15-126 and DI15-127 and 16 mg/L for strain DI15-128. These values are moderately elevated when compared with the activity of isavuconazole against Rhizopus spp. (MIC of 0.25–1.0 mg/L)6 and are concordant with previous reports of isavuconazole activity against Mucor spp.15,16
Next, we conducted pharmacokinetic studies. A previous study demonstrated that oral–gastric doses of the prodrug isavuconazonium sulphate over a range of 10–640 mg/kg produced serum peak isavuconazole levels of 0.51–25.4 mg/L and an elimination half-life of 1–5 h.17 Hence, we administered the prodrug isavuconazonium sulphate at 80, 110, 215 and 400 mg/kg three times daily for 4 days and determined the serum isavuconazole levels 4 h after the last dose. Although all doses demonstrated a significant increase in serum isavuconazole levels, only doses of 110, 215 and 400 mg/kg showed isavuconazole levels between ∼10 and ∼30 mg/L. Such levels are in excess of the MIC against Mucor (Figure 1a).
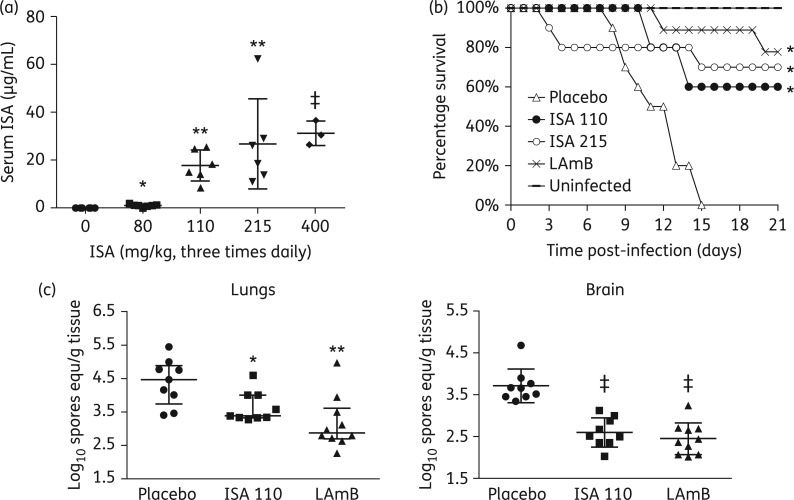
Figure 1.
Isavuconazole (ISA) is as effective as a high dose of LAmB in treating murine mucormycosis due to M. circinelloides f. jenssenii. (a) Serum ISA concentration in mice (n = 6 mice per group) treated with different doses of the antifungal agent given three times daily for 4 days. Sera were collected 4 h after the last dose. (b) Survival of neutropenic mice (n = 10 per group except LAmB-treated mice, which had 9 mice) infected intratracheally with M. circinelloides f. jenssenii (instilled inoculum of 1.7 × 103 per mouse) and treated with ISA (given orally) at 110 mg/kg ‘three times daily’ (ISA 110), ISA at 215 mg/kg three times daily (ISA 215) or LAmB (given iv) at 15 mg/kg once daily. *P < 0.005 versus placebo-treated mice. (c) Lungs and brain fungal burden of mice (n = 9 for placebo- or ISA-treated mice and 10 for LAmB-treated mice) infected with M. circinelloides f. jenssenii, treated on day +1 through day +4 and sacrificed on day +7, relative to infection. Data are expressed as median ± IQR. *P < 0.02 versus placebo-treated mice, **P < 0.05 versus placebo and ISA-treated mice and ‡P < 0.0001 versus placebo-treated mice.
Because the high dose of 400 mg/kg three times daily demonstrated toxicity (three of the mice expired) without a benefit in increasing isavuconazole serum level versus the 215 mg/kg dose (Figure 1a), we compared the therapeutic efficacy of the prodrug at 110 and 215 mg/kg three times daily with that of LAmB. Mice infected with M. circinelloides f. jenssenii were protected with isavuconazonium sulphate at 110 or 215 mg/kg three times daily to comparable levels seen with the high-dose LAmB at 15 mg/kg once daily (60% and 70% survival for 110 and 215 mg/kg isavuconazole, respectively, versus 78% survival for LAmB treatment) (Figure 1b).
Because isavuconazole increased the survival rate of mice infected with M. circinelloides f. jenssenii, the effect of drug treatment on the tissue fungal burden in target organs was determined. Only the 110 mg/kg dose was tested as it had similar pharmacokinetics and survival to the higher dose of 215 mg/kg. Treatment of mice with the isavuconazole prodrug resulted in ∼1 log decreases in fungal burdens in lungs and brain compared with placebo-treated controls. LAmB treatment resulted in a significantly better reduction in cfu in the lungs when compared with placebo or isavuconazole 110 mg/kg and was comparable to the reduction elicited by isavuconazole in the brain (Figure 1c).
In vivo activity of combination therapy of isavuconazole and micafungin is similar to monotherapy
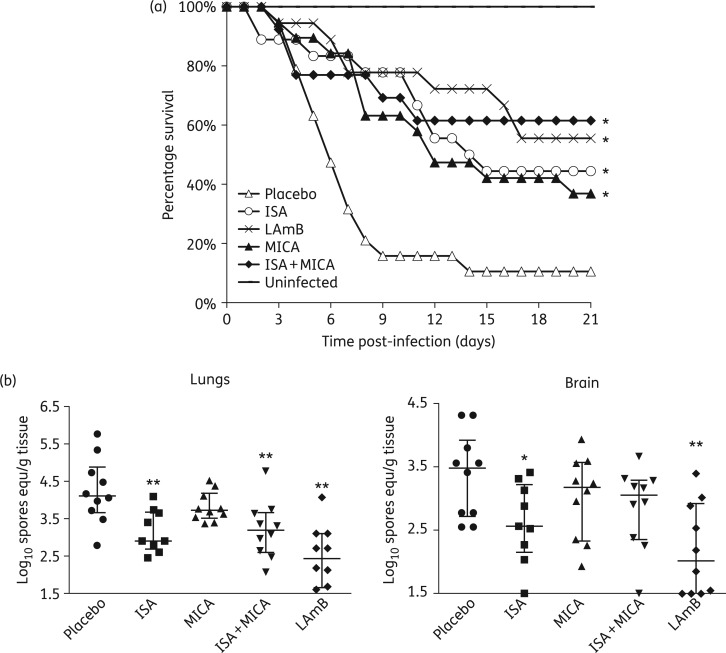
Due to the poor clinical outcome of mucormycosis, antifungal combination therapies are frequently used.18 There has been experimental evidence using the diabetic and the neutropenic mouse models9 and case reports10 showing the benefit of adding an echinocandin to lipid formulation amphotericin B in treating mucormycosis. Consequently, we evaluated the role of combining micafungin with isavuconazole. Mice were infected with R. delemar and treated as above with isavuconazonium sulphate 215 mg/kg three times daily, micafungin 1 mg/kg once daily or a combination of both and compared with LAmB 15 mg/kg once daily. All monotherapy arms resulted in a comparable significant enhancement of 40%–60% survival when compared with placebo-treated mice. Although mice treated with isavuconazole + micafungin had significantly enhanced survival compared with placebo-treated mice, the combination therapy was not better than treatment with monotherapy (Figure 2a). These results were mirrored in the tissue fungal burden results with the exception of mice treated with micafungin, which did not show any reduction in cfu (Figure 2b). The lack of tissue fungal burden reduction with micafungin despite its enhancing survival time, indicates a mechanism of action of echinocandins against mucormycosis that is independent of a direct killing of the fungus, which might be caused by some type of beneficial immunomodulatory effect.19 Further, the lack of benefit in the combination therapy appears to be in agreement with a recently published article showing that initial combination therapy does not impact survival of patients with mucormycosis with haematological malignancies.18 Finally, the discrepancy between the finding of the combination studies presented here and our previous studies describing a benefit of using an echinocandin (i.e. caspofungin acetate, anidulafungin or micafungin) + lipid formulation amphotericin B (i.e. amphotericin B lipid complex or LAmB) in the diabetic and neutropenic mouse models9,20 could be due to the differences in drug classes tested (i.e. echinocandins + lipid formulation amphotericin B versus echinocandins + azoles) or it could be due to differences in the models used (iv versus intratracheal infection). In conclusion, our findings support the wider use of isavuconazole against mucormycosis in the setting of neutropenia. However, in this model, combination treatment with isavuconazole + micafungin did not demonstrate enhanced efficacy over monotherapy with either isavuconazole or LAmB.
Figure 2.
Isavuconazole (ISA) + micafungin (MICA) combination therapy is not better than either drug alone in treating murine mucormycosis due to R. delemar. (a) Survival of neutropenic mice [n = 19 for placebo-, 18 for ISA- (given orally) (215 mg/kg three times daily), 19 for MICA- (administered by ip injection) and 13 for ISA + MICA-treated mice, from two separate experiments that included all treatment regimens] infected intratracheally with R. delemar (average inhaled inoculum of 1.3 × 103 per mouse). Treatment started on day +1 and lasted through day +4, relative to infection. *P < 0.02 versus placebo-treated mice. (b) Lungs and brain fungal burden of mice (n = 10 per group) infected with R. delemar (average inoculum of 4.2 × 103 per mouse), treated on days 0 (8 h post-infection) through +2 and sacrificed on day +3, relative to infection. Data are expressed as median ± IQR. *P < 0.03 versus placebo-treated mice and **P < 0.03 versus placebo- and MICA-treated mice.
Funding
This work was supported by Public Health Service grant R01 AI063503 and a research and educational grant from Astellas Pharma USA to A. S. I.
Transparency declarations
N. P. W. and A. S. I. served on the Scientific Advisory Boards of Astellas Pharma USA. N. A. is an employee of Astellas Pharma USA. All other authors: none to declare.
Acknowledgements
This work was presented at the First ASM Microbe, Boston, MA, USA, 2016 (oral presentation no. 5844).
Research described in this manuscript was conducted in part at the research facilities of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center.
References
- 1. Sugar AM. Agent of mucormycosis and related species. In: Mandell G, Bennett J, Dolin R, eds. Principles and Practices of Infectious Diseases. 4th edn New York: Churchill Livingstone; 1995: 2311–21. [Google Scholar]
- 2. Husain S, Alexander BD, Munoz P et al. . Opportunistic mycelial fungal infections in organ transplant recipients: emerging importance of non-Aspergillus mycelial fungi. Clin Infect Dis 2003; 37: 221–9. [DOI] [PubMed] [Google Scholar]
- 3. Roden MM, Zaoutis TE, Buchanan WL et al. . Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005; 41: 634–53. [DOI] [PubMed] [Google Scholar]
- 4. Martin de la Escalera C, Aller AI, Lopez-Oviedo E et al. . Activity of BAL 4815 against filamentous fungi. J Antimicrob Chemother 2008; 61: 1083–6. [DOI] [PubMed] [Google Scholar]
- 5. Guinea J, Pelaez T, Recio S et al. . In vitro antifungal activities of isavuconazole (BAL4815), voriconazole, and fluconazole against 1007 isolates of zygomycete, Candida, Aspergillus, Fusarium, and Scedosporium species. Antimicrob Agents Chemother 2008; 52: 1396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luo G, Gebremariam T, Lee H et al. . Isavuconazole therapy protects immunosuppressed mice from mucormycosis. Antimicrob Agents Chemother 2014; 58: 2450–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marty FM, Ostrosky-Zeichner L, Cornely OA et al. . Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case–control analysis. Lancet Infect Dis 2016; 16: 828–37. [DOI] [PubMed] [Google Scholar]
- 8. Ibrahim AS, Bowman JC, Avanessian V et al. . Caspofungin inhibits Rhizopus oryzae 1,3-β-d-glucan synthase, lowers burden in brain measured by quantitative PCR, and improves survival at a low but not a high dose during murine disseminated zygomycosis. Antimicrob Agents Chemother 2005; 49: 721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ibrahim AS, Gebremariam T, Fu Y et al. . Combination echinocandin-polyene treatment of murine mucormycosis. Antimicrob Agents Chemother 2008; 52: 1556–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reed C, Bryant R, Ibrahim AS et al. . Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis 2008; 47: 364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi—Second Edition: Approved Standard M38-A2. CLSI, Wayne, PA, USA, 2008. [Google Scholar]
- 12. Luo G, Gebremariam T, Lee H et al. . Efficacy of liposomal amphotericin B and posaconazole in intratracheal models of murine mucormycosis. Antimicrob Agents Chemother 2013; 57: 3340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wiederhold NP, Pennick GJ, Dorsey SA et al. . A reference laboratory experience of clinically achievable voriconazole, posaconazole, and itraconazole concentrations within the bloodstream and cerebral spinal fluid. Antimicrob Agents Chemother 2014; 58: 424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ibrahim AS, Avanessian V, Spellberg B et al. . Liposomal amphotericin B, and not amphotericin B deoxycholate, improves survival of diabetic mice infected with Rhizopus oryzae. Antimicrob Agents Chemother 2003; 47: 3343–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verweij PE, Gonzalez GM, Wiedrhold NP et al. . In vitro antifungal activity of isavuconazole against 345 mucorales isolates collected at study centers in eight countries. J Chemother 2009; 21: 272–81. [DOI] [PubMed] [Google Scholar]
- 16. Arendrup MC, Jensen RH, Meletiadis J. In vitro activity of isavuconazole and comparators against clinical isolates of the Mucorales order. Antimicrob Agents Chemother 2015; 59: 7735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lepak AJ, Marchillo K, Vanhecker J et al. . Isavuconazole pharmacodynamic target determination for Candida species in an in vivo murine disseminated candidiasis model. Antimicrob Agents Chemother 2013; 57: 5642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kyvernitakis A, Torres HA, Jiang Y et al. . Initial use of combination treatment does not impact survival of 106 patients with haematologic malignancies and mucormycosis: a propensity score analysis. Clin Microbiol Infect 2016; 22: 811.e1–8. [DOI] [PubMed] [Google Scholar]
- 19. Spellberg B, Walsh TJ, Kontoyiannis DP et al. . Recent advances in the management of mucormycosis: from bench to bedside. Clin Infect Dis 2009; 48: 1743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spellberg B, Fu Y, Edwards JE Jr et al. . Combination therapy with amphotericin B lipid complex and caspofungin acetate of disseminated zygomycosis in diabetic ketoacidotic mice. Antimicrob Agents Chemother 2005; 49: 830–2. [DOI] [PMC free article] [PubMed] [Google Scholar]