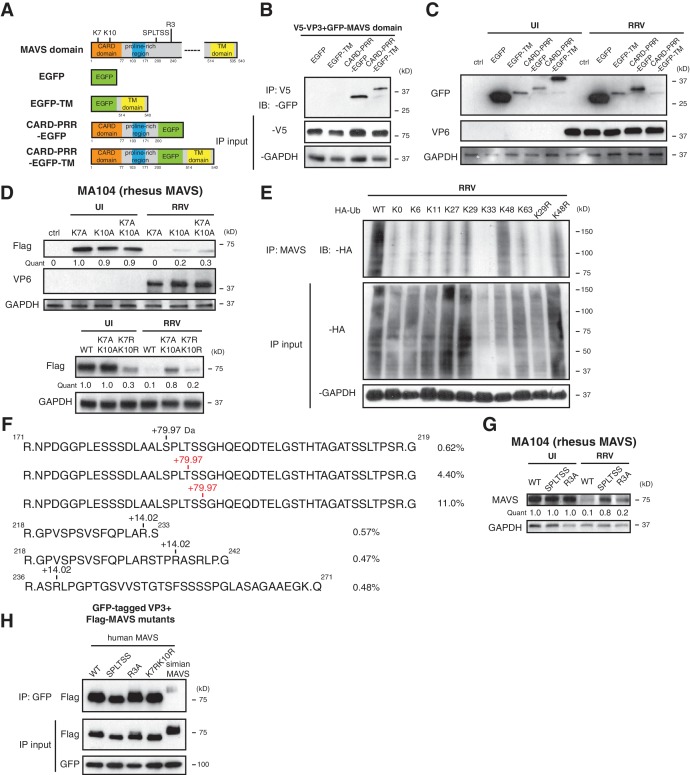
Figure 4. Phosphorylation mediates MAVS degradation during RV infection.
(A) Schematic diagram of MAVS and chimeric EGFP-MAVS proteins with defined domains illustrated in colors. Two lysines within the CARD domain and novel post-translational modifications (PTMs) are pointed out (left panel). (B) MA104 cells were transfected with indicated MAVS plasmids for 48 hr with or without RRV infection (MOI = 1) for the last 12 hr. The lysates were harvested and examined by western blot. (C) MA104 cells were co-transfected with GFP-tagged MAVS domain mutants and V5-tagged RRV VP3 for 48 hr and lysates were harvested for immunoprecipitation using anti-V5 antibody. (D) MA104 cells were transfected with indicated GFP-tagged MAVS mutants for 48 hr with or without RRV infection (MOI = 1) for the last 12 hr. The levels of MAVS and viral protein VP6 expression were measured by western blot. (E) MA104 cells were transfected with WT or indicated lysine-mutants of HA-tagged ubiquitin for 48 hr, infected with RRV (MOI = 1) for the last 12 hr and harvested for immunoprecipitation using an anti-MAVS antibody. The levels of ubiquitin-conjugated MAVS were measured using an anti-HA antibody. (F) Mass spectrometry analysis of PTMs on MAVS, immunoprecipitated from RRV-infected MA104 lysates at six hpi. Numbers indicate the mass increase (+79.97: phosphorylation;+14.02: methylation). The novel PTMs identified in this study are highlighted in red. The percentage of phosphorylation and methylation modification in total MAVS proteins is presented as %. (G) MA104 cells were transfected with WT or MAVS mutants (SPLTSS: SPLTSS motif mutated to six alanines; R3A: R232, R236, R239 mutated to three alanines) for 48 hr with or without RRV infection (MOI = 1) for the last 12 hr. The levels of MAVS were measured by western blot. (H) HEK293 cells were co-transfected with GFP-tagged Wa-VP3 and WT or MAVS mutants for 48 hr and treated with MG132. Lysates were harvested for immunoprecipitation using anti-GFP antibody and probed for MAVS levels. For all figures except (e) and (f), experiments were repeated at least three times with similar results. Experiment in (e) was performed twice and (f) was performed once.