Abstract
Adipose tissue dysfunction underlies the pathogenesis of metabolic disease. The metrics used to quantify adiposity and its association with metabolic disease, including body mass index, have limitations with important clinical implications. An understanding of the molecular and cellular mechanisms by which adipose tissue regulates systemic metabolism and contributes to metabolic disease will lead to next-generation adipose tissue-based therapy.
Keywords: Adipose tissue, Body mass index, Obesity, Metabolic disease, Cell stress responses
Measuring adiposity
“To measure is to know.”
−Lord Kelvin
“Measure what is measurable, make measurable what is not.”
−Galileo Galilei
A cure for obesity?
In 1994 Jeffrey Friedman at the Rockefeller Institute cloned the leptin gene [1]. His discovery was met with much excitement as leptin, a satiety-inducing hormone secreted by adipose tissue, was thought to be the long-sought cure for obesity. Indeed, subsequent clinical trials of exogenous leptin therapy in rare humans with genetic leptin deficiency demonstrated dramatic efficacy [2]. However, further study revealed that the vast majority of obese humans do not suffer from genetic leptin deficiency but instead manifest hyperleptinemia with hypothalamic resistance to leptin’s satiety-inducing effects, rendering exogenous leptin therapy ineffective [3]. While leptin has yet to provide transformative therapy for obesity, Friedman’s discovery dramatically shifted attention to adipose tissue as a central mediator of metabolism and the site of genesis of metabolic disease.
Three large U.S. population-based studies—the National Health and Nutrition Examination Survey, the National Health Interview Survey, and the Behavioral Risk Factor Surveillance System—demonstrate that from 1970 to 2005 mean body mass index (BMI) increased from 25.5 to 28.5 and the prevalence of obesity more than doubled, now afflicting over a third of the U.S. population (Fig. 1A) [4–6]. In 2013 and 2014, over 50% of U.S. adults had a history of or presently had obesity, suggesting that static prevalence underestimates the magnitude of the problem [7]. The public health burden of this epidemic is immense. The prevalence of type 2 diabetes in the United States is estimated to be between 7% and 12% [7,8] and will exceed 30% by 2050 [9]. Diabetes alone currently accounts for >11% of deaths and >20% of healthcare expenditures in the United States [7,8]. Lifespan is reduced by as much as 3, 6, and 13 years in overweight, obese, and severely obese subgroups, respectively [10]. The metabolic disease epidemic represents one of our greatest societal challenges.
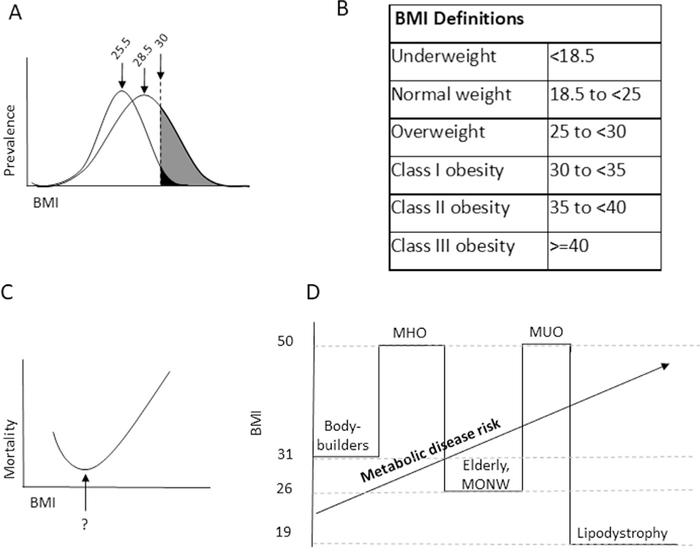
Fig. 1.
Associations of body mass index (BMI) with metabolic disease. (A) Schematic representative BMI prevalence curves in leptogenic and obesogenic environments. In a leptogenic environment, the obese phenotype is restricted to a relatively narrow BMI range, as food scarcity prevents humans with a propensity to obesity to manifest this phenotype. As nutrient availability increases in an obesogenic environment, a relatively modest increase in mean BMI may be associated with a large increase in obesity prevalence as a result of a widening and rightward shift of the BMI population distribution curve, with blossoming of the obese phenotype among susceptible individuals. Mean BMIs shown for each curve are those associated with National Health and Nutrition Examination Survey data from 1970 (25.5) and 2004 (28.5). (B) The relationship between BMI and long-term mortality. The nadir of this curve remains debated but recent epidemiologic data support that it lies between BMI 20 and 25. Similar J-shaped curves describe the relationship between many phenotypic features and disease/mortality risk (e.g., blood pressure). (C) World Health Organization-Centers for Disease Control weight definitions. (D) Limitations in BMI as a metric for metabolic disease risk result from epidemiologic and biologic factors. BMI does not distinguish between adipose and muscle mass, limiting its predictive power for disease risk in athletes and the elderly. Fundamental differences in biology, such as patients with lipodystrophy and metabolically unhealthy obesity, metabolically healthy obesity, or metabolically obese normal weight phenotypes, also limit accuracy.
BMI: a brief history
We measure obesity, or more precisely adiposity, to predict the risk of metabolic disease-related morbidity and mortality. Measuring adiposity is fraught with challenges, and its association with disease is complex. As such, the metrics and definitions applied to obesity and their clinical implications are hotly debated in professional and public forums.
For over a century, students of anthropometry, the study of quantitative features of body morphology, have sought a single metric that accurately normalizes weight to height, permitting measurement of weight independent of height in large populations. Weight approximates volume, which varies with the cube of linear dimension and therefore cannot be normalized to height using a simple weight/height ratio. If weight scaled perfectly with volume, weight/height3 (the Ponderal index) [11] would provide accurate normalization. However, due to variability in body shape and composition, weight does not scale perfectly with volume. Lambert Adolphe Jacques Quetelet, 19th century Belgian polymath, was the first to empirically demonstrate this. Quetelet studied height and weight distributions in large populations and found that only when expressed as weight/height2 (the Quetelet index) did weight assume a normal Gaussian distribution among individuals of varying heights, permitting calculation of an average population weight independent of height [12]. Notably, Quetelet was not specifically interested in medicine or obesity and never intended his index to be used as an individual health metric. Rather, his research studied the interface of human physiology, sociology, and morality. His ambitious goal was to use anthropometric, physical, moral, and intellectual characteristics of l’homme moyen, the average man, which he conceived as the epitome of human development, to define social and moral behaviors of society as a whole [13]. While Quetelet was lauded for his contributions to mathematics, statistics, astronomy, and sociology during his lifetime [14], his index received little attention in 19th century clinical medicine.
In the early 20th century, in response to an increasing realization of the adverse health consequences of obesity, the U.S. insurance industry began using actuarial tables that defined “ideal” weight based on average weights for individuals grouped into different height ranges to estimate mortality risk of its beneficiaries. These methods, while widely used [15,16], were criticized, as their predictive power was limited. In the 1970s, in separate reports, Charles Florey at Yale University and Ancel Keys at the University of Minnesota used data from the Framingham study and other population-based studies to revisit the goal of identifying a more accurate anthropometric measure for adiposity. Florey and Keys demonstrated that the Quetelet index, compared with simple height/weight ratios or to the Ponderal index, predicted adiposity measured by skinfold thickness most accurately over a wide range of variable heights and weights. Keys renamed Quetelet’s index the “body mass index,” which was adopted by the insurance industry and has since achieved widespread use in health outcomes research [17,18].
Limitations of BMI
Several large epidemiologic studies have led to current BMI-based definitions of overweight and obesity agreed upon by the World Health Organization, the Centers for Disease Control, and the National Heart, Lung, and Blood Institute (Fig. 1B) [19]. These studies consistently demonstrate that the relationship between BMI and disease and mortality risk is described by a J-shaped curve with increasing risk at extremes of BMI (Fig. 1C). Nonetheless, controversy persists regarding the nadir of this curve, which early studies placed in the overweight BMI range (25–29.9), giving rise to the “fat-but-fit” hypothesis [20,21]. While persisting in the lay press, recent data argue against this concept. Early reports failed to control for confounders, most importantly smoking and chronic disease, which are associated with increased mortality in lower BMI patients, thus contaminating comparator groups and artificially lowering relative mortality for higher BMI patients without these risk factors. Recent meta-analyses restricted to patients with no history of smoking or chronic disease demonstrate that the nadir of mortality lies in the BMI 20 to 25 range [22–25]. Furthermore, most studies stratify BMI into multiples of 5. Finer resolution of optimal BMI remains elusive but may lie in the 20 to 23 range [22]. This lack of resolution influences risk estimates for higher BMI categories, as correlations between overweight BMI and adverse clinical outcomes weaken significantly and may be masked when using comparator-referent groups spanning a larger BMI range (e.g., 18–25) relative to referent groups in a narrow BMI range (e.g., 21–23). In other words, how we define ideal weight is as important as how we define obesity, as these definitions are inextricably co-dependent. These uncertainties aside, a preponderance of data suggests that for the majority of the population, BMI >25 imparts increased health risk.
BMI is by no means a perfect measure of adiposity. Its most significant limitation is an inability to distinguish between adipose and muscle mass. Body builders may have BMIs >30 despite very low percent body fat, while elderly patients with decreased muscle mass may have increased adiposity and disease risk at normal BMI (Fig. 1D). Ethnicity also influences the relationship between BMI and disease. Black people have higher fat-free mass than white people and develop metabolic dysfunction at relatively higher BMI [26]. Asian people in contrast develop metabolic disease at lower BMI, due to selection for diabetogenic single nucleotide polymorphisms in Asian populations, such that obesity is defined as BMI > 25 in Japan and >28 in China [27,28]. Finally, overweight or obese BMI may be associated with decreased mortality in patients with chronic diseases, in whom lower BMI, rather than a sign of metabolic health, may be a marker for malnutrition, a phenomenon termed the obesity paradox. BMI is not a one-size-fits-all metric.
Further complicating analysis, BMI displays quantitatively and qualitatively different associations with various metabolic diseases, and when considered as a constellation of pathologies, the picture becomes even more confusing. The National Health and Nutrition Examination Survey III defines metabolic syndrome as > 2 of the following criteria: hypertension, hyperlipidemia, fasting blood glucose >100, hypoglycemic medication use, C-reactive protein >90th percentile, and homeostatic model assessment >90th percentile [29]. Other organizations propose alternative definitions, and consensus has yet to be achieved.
Next-generation metrics
The complexities of BMI’s correlations with disease have important clinical implications. Recent data refute the fat-but-fit hypothesis and support counseling of patients in overweight as well as obese BMI categories. Furthermore, while the National Institutes of Health criteria for bariatric surgery established in 1991 include Class III obesity or Class II obesity with metabolic disease, emerging data demonstrate that diabetic patients with Class I obesity also benefit [30], leading to consideration of expanding bariatric surgery candidacy. This shifting landscape also applies to Asia, where BMI thresholds for surgical candidacy are 2 to 3 kg/m2 lower [28,30], reflecting the influence of ethnicity on adiposity and disease. Finally, identification of high-risk patient subpopulations is critical for optimal allocation of care, prompting a search for high-fidelity metrics for adiposity and disease risk. Waist circumference and waist-hip ratio may better predict disease risk than BMI, reflecting a more accurate estimation of visceral and truncal adiposity, tissue depots more strongly associated with disease. Conversely, thigh circumference measures subcutaneous and extremity adiposity, depots that correlate inversely with metabolic disease, and is thus a strong predictor of metabolic health. Application of these alternative anthropometric tools is increasing as data evolves. X-ray absorptiometry, bioelectrical impedance, air plethysmography, computed tomography, and magnetic resonance imaging accurately measure adiposity but are limited by cost, logistics, and risks. Next-generation technologies study functional alterations in adi-pose tissue and include heavy isotope magnetic resonance spectroscopy, optical spectroscopy, microwave radiometry, and positron emission tomography-computed tomography, techniques capable of measuring specific metabolic pathways in adipose tissue. These evolving technologies depend on a growing understanding of molecular and cellular mechanisms of adipose tissue dysfunction in the context of metabolic disease.
Exceptions to the rule
Obesity is a heterogeneous phenotype with significant variability in age of onset, triggers (e.g., pregnancy), body habitus (e.g., android versus gynoid), and, most importantly, relationship with metabolic disease. A subset of obese patients appears to be protected from disease, a phenotype termed metabolically healthy obesity, comprising 10% to 25% of the obese population [31]. Conversely, some patients manifest metabolic abnormalities despite BMI in the normal range, a phenotype termed metabolically obese but normal weight [32]. Ethnicity influences the imperfect relationship between adiposity and metabolic disease, as the prevalence of metabolically obese but normal weight among patients with normal BMI ranges from 20% in white people to up to 40% in South Asian people [32,33]. This phenotypic heterogeneity demonstrates that the imperfect correlation between adiposity and metabolic disease is not due solely to epidemiologic confounders or limitations in adiposity metrics but rather has its basis in biology (Fig. 1D). The biologic basis of metabolically protected and at-risk subpopulations represents one of the most crucial unanswered questions in obesity research. To a large extent, the answer lies within adipose tissue.
Adipose tissue and metabolic disease pathogenesis
“Everything in excess is opposed to nature. Repletion, carried to extremes, is perilous.”
−Hippocrates
Nutrient toxicity
Patients with rare congenital lipodystrophy syndromes lack adipose tissue, have BMIs <20, and develop diabetes, steatosis, and hyperlipidemia, a metabolic phenotype similar to obesity. The parallels between lipodystrophy and obesity speak to adipose tissue’s dominant function as a nutrient buffer, protecting other tissues from nutrient toxicity. In lipodystrophy, the absence of adipose tissue causes metabolic disease, as other tissues are exposed to excess nutrients normally sequestered in adipose tissue. In obesity, systemic toxicity results from failure of adipose tissue’s nutrient buffering capacity due to maladaptive adipocyte responses to nutrient excess. The concept of nutrient toxicity has its basis in the biochemical nature of nutrients, which carry labile, high-energy electrons capable of transferring energy to other molecules. While harnessed by cells to carry out useful work, the very properties that make nutrients such efficient energy carriers allow them to inflict widespread damage if uncontrolled. As a result, cells have evolved mechanisms to control reactions in which nutrients participate.
In the early 20th century, 2,4-dinitrophenol (DNP), a compound used in the clothing dye and munitions industries, was observed to cause weight loss in employees. DNP was subsequently marketed as a weight loss drug but was discontinued after being associated with cases of fatal hyperthermia [34,35]. DNP mediates weight loss by interrupting oxidative phosphorylation, a fundamental metabolic reaction in all eukaryotic cells. This early foray into obesity pharmacotherapy highlights the hazards of manipulating energy homeostasis, a lesson revisited many times over the past century, as multiple weight loss drugs have been introduced with much excitement, only to be withdrawn due to prohibitive toxicity.
The coupling of electron transport to oxidative phosphorylation within mitochondria, a central metabolic process of aerobic life, is one of many mechanisms evolved by cells to harness nutrients safely (Fig. 2A). DNP acts as an ionophore that permits passive flow of protons through the inner mitochondrial membrane, short circuiting the proton gradient and releasing energy as heat rather than using it for ATP (adenosine triphosphate) synthesis. Spatial coupling of oxidative phosphorylation and electron transport localizes and organizes enzymes and substrates necessary for this complex set of high-energy reactions within the inner mitochondrial membrane, allowing them to proceed efficiently, which could not occur in a cytoplasmic soup dependent only on Brownian motion. This spatial organization depends on a membrane-based chemical gradient (in this case, protons) as an energy source, a fundamental feature of cell-based life. Finally, spatial localization sequesters high-energy molecules (in this case, within mitochondria), limiting their involvement to reactions that benefit the cell (e.g., oxidative phosphorylation) while preventing them from “getting loose” and participating in damaging reactions (e.g., DNA hydrolysis). Sequestration of nutrients in specialized organelles, such as mitochondria, is a ubiquitous feature of eukaryotic cells. This sequestration occurs at the tissue level as well, as metazoan organisms have evolved tissues specifically designed to control these potentially dangerous molecules. Adipose tissue is the epitome of this evolutionary design process.
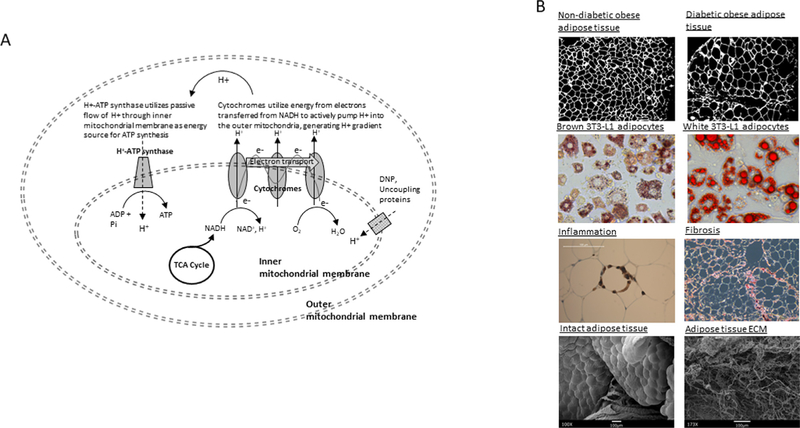
Fig. 2.
Nutrient sequestration and tissue manifestations of its failure. (A) Coupling of oxidative phosphorylation and electron transport. ATP synthesis by the H+−ATP synthase embedded within the inner mitochondrial membrane (oxidative phosphorylation) is driven by transfer of nutrient-derived high-energy electrons down a chain of cytochrome proteins embedded in the same membrane (electron transport). Cytochromes transfer high-energy electrons derived from NADH+ to O2, generating H2O and CO2, while simultaneously using their energy to pump protons across the inner mitochondrial membrane, creating a proton gradient that is used by the H+ ATP synthase as an energy source to synthesize ATP. NADH+gains these high-energy electrons from carbon-based metabolites generated by glycolytic breakdown of nutrients (i.e., glucose) in the tricarboxylic acid cycle. The chemical agent 2,4-dinitrophenol acts as an ionophore, allowing passive leak of protons back into the inner mitochondrial, short circuiting the system and allowing the mitochondrial engine to effectively “run in neutral”; uncoupling proteins similarly dissipate the proton gradient in a regulated manner, and are especially active in brown adipocytes. (B) Histologic manifestations of adipose tissue failure. Top: human adipose tissue from nondiabetic (left) and diabetic (right) patients demonstrating increased adipocyte hypertrophy in metabolic disease. Middle top: murine 3 T3 L1 adipocytes differentiated in conditions that induce brown (left) or white (right) adipocyte phenotypes and stained with lipid-avid Oil Red-O dye; note tendency toward small, multilocular lipid dropets in brown adipocytes, and larger, unilocular lipid droplets in white adipocytes; transcripts levels of uncoupling proteins are increased 100-fold in brown compared to white adipocytes (data not shown). Middle bottom: human adipocyte surrounded by macrophages in a ‘crown-like structure’, in which macrophages target adipocytes undergoing cell stress or apoptosis (left); adipose tissue stained with antiCD68 antibody; human adipose tissue stained with collagen-avid dye Sirius Red, demonstrating fibrotic bands and peri-adipocyte fibrosis (right). Bottom: scanning electron micrographs of intact human adipose tissue (left) and adipose tissue after decellularization (right), in which all cells including adipocytes are removed, leaving behind only an extensive fibrotic extracellular matrix. Note: in human patients, human adipose tissues were collected with patient consent and approval by institutional review boards of University of Michigan and Ann Arbor Veterans Administration Hospital.
Adipocyte failure
Lipids and their downstream metabolites are particularly high-energy molecules and, as such, the preferred energy storage vehicle for adipocytes. Up to 97% of adipocyte volume is composed of a unilocular lipid droplet, traffic in and out, which is tightly regulated by multiple fatty acid binding/transfer proteins, mutations in which are among the many genetic polymorphisms that contribute to common obesity and congenital lipodystrophies [36]. This sophisticated machinery permits adipocytes to safely store large amounts of lipid, more than any other mammalian cell. However, even adipocytes have their limits that, when reached, lead to metabolic disease.
Hypertrophy is the initial response of adipocytes to nutrient excess, an adaptive mechanism that increases nutrient buffering capacity. Adipocytes are capable of hypertrophy to diameters >200 μm, among the largest human cells. Hypertrophy is adaptive in early stages, as lean patients with larger adipocytes manifest increased insulin sensitivity [37]. Adipocyte hypertrophy beyond a certain threshold, in contrast, correlates strongly and directly with metabolic disease in obese humans [38]. Hypertrophy beyond a cell diameter of 100 μm, the diffusion distance of oxygen, is postulated to induce a state of cellular hypoxia, one of a number of external stressors that triggers a complex set of stress responses common to all cells. Cell stress responses, including endoplasmic reticulum stress and oxidative stress, are initiated by not only hypoxia, but also by infection, trauma, ischemia, reactive oxygen species, and, importantly, nutrient excess [39]. Cell stress responses activate transcriptional programs that initially limit nutrient processing and energy production to protect individual cells. If stress stimuli persist, stress responses go on to induce programmed apoptotic cell death to limit global tissue damage. Cell stress responses are adaptive in transient stress, limiting tissue damage and hastening resolution of physiologic insults, but become maladaptive in the face of chronic stress, such as longstanding infection, or, as in obesity, chronic nutrient excess.
Cell stress responses are intertwined with other fundamental cellular processes, including inflammation and metabolism. The immune system is highly energy intensive, consuming up to 20% of total body energy requirements [40]. Inflammatory cytokines expressed by immune cells induce insulin resistance in most other cells by downregulating expression of insulin signaling molecules, decreasing glucose utilization by nonimmune tissues to preserve energy for the immune system. This interplay between immunity and metabolism, termed metainflammation, occurs in all tissues, but in obesity has its genesis in adipose tissue [41]. Adipocyte apoptosis resulting from nutrient excess- and hypoxia-induced cell stress generates an inflammatory response, as macrophages and other immune cells infiltrate adipose tissue to scavenge dead and dying adipocytes. Inflammatory mediators expressed by infiltrating immune cells in turn induce insulin resistance in surviving adipocytes, further impairing adipose tissue metabolism. As inflammation persists, a fibrotic response is generated in which collagens and other extracellular matrix proteins are deposited in excess, limiting adipocyte hypertrophic capacity, exacerbating overflow, and leading to adipose tissue failure (Fig. 2B) [42].
Nutrient overflow
Once adipocytes reach their hypertrophic limits, lipids overflow into the systemic circulation, causing toxicity in tissues not adapted to processing high levels of these bioenergetics molecules. As visceral adipose tissue reaches its nutrient storage capacity, free fatty acids flood the liver via the portal venous system (portal overflow), causing hepatic steatosis, an early manifestation of systemic disease. In >30% of humans, steatosis progresses to hepatocyte cell stress, inflammation, and fibrosis, hallmarks of steatohepatitis, susceptibility to which is linked to a number of genetic polymorphisms involving genes that regulate hepatic cell stress responses [43]. As obesity progresses, peripheral overflow beyond the liver ensues, with lipotoxi-city affecting all tissues. Skeletal muscle myocytes, which account for up to 75% of whole-body glucose utilization [44,45], shift energy consumption away from glucose and toward lipids in response to increased lipid delivery, resulting in reduced glucose uptake by muscle, a pathognomonic feature of peripheral insulin resistance. As in adipose tissue, skeletal muscle insulin resistance is exacerbated by inflammation in response to myocyte apoptosis caused by nutrient excess-triggered cell stress processes. Similar events transpire in other tissues. Asthma, atopic disease, kidney disease, cancer, vascular disease, and arthritis all have increased incidence in obesity, with cell stress and inflammation evident in corresponding tissue beds.
Adipose tissue heterogeneity
Adipose tissue manifests striking heterogeneity. Subcutaneous adipose tissue (SAT) constitutes >85%, and visceral adipose tissue (VAT) <10%, of total body adipose stores [46], but excess VAT is nonetheless a much stronger risk factor for metabolic disease than excess SAT, which may in fact exert a protective metabolic influence [47,48]. Preferential drainage of VAT’s venous effluent into the portal venous system with disproportionate effects on hepatic metabolism may partly explain its relationship to disease (the portal theory), but qualitative functional differences also contribute, including higher lipolytic capacity and increased inflammation [49]. The distinction between VAT and SAT does not fully capture the anatomic complexity of these depots, which are further subdivided into omental, mesenteric, and retroperitoneal VAT subdepots, and truncal and extremity, and superficial and subfascial SAT subdepots, each with distinct phenotypes and disease associations. Adipose tissue is also found in the hands and feet, face and orbit, bone marrow, and peri-vascular and -organ spaces. Adipose tissue is best considered a group of related but functionally distinct organs.
Humans exhibit significant variability in anatomic sites of adipose tissue deposition. Men tend to accumulate excess adipose tissue in the visceral compartment, while women preferentially accumulate subcutaneous adipose tissue. With aging, both men and women shift adipose tissue deposition from subcutaneous to visceral compartments. As such, male sex and age are independent risk factors for metabolic disease [50]. A tendency toward gynoid obesity may in part be due to elevated expression of lipoprotein lipase in SAT, an enzyme critical to lipid import in adipocytes [51]. Similar variability may underlie nongender-based differences in disease risk, as expression of genes involved in glucose uptake and lipogenesis in SAT is increased in metabolically healthy obese humans [52]. The genes that regulate differential expansion of adipose tissue depots are not yet fully described, but candidates are emerging; mice deficient in Fyn, a Src family kinase with diverse functions, manifest a metabolically healthy obesity phenotype characterized by increased expansion of SAT and decreased VAT expansion [53]. Polymorphisms in genes like Fyn may regulate differences in nutrient partitioning to different adipose tissue depots in humans as well, contributing to variable disease susceptibility, and suggesting the possibility of manipulation of adipose tissue depot partitioning as a therapeutic strategy.
Adipose tissue displays cellular and anatomic heterogeneity. VAT and SAT are white adipose tissues, which constitute the vast majority of adipose tissue in adult humans and range from <7% of total body volume in elite athletes to >50% in obesity [54,55]. White adipocytes exhibit cellular metabolism geared toward energy storage. Brown adipose tissue (BAT) in contrast comprises only .05% of total body mass in humans. Brown adipocytes express high levels of uncoupling proteins that uncouple oxidative phosphorylation from electron transport, generating heat rather than ATP. BAT is thus wired for energy expenditure and in mice and human neonates serves as a heat source by engaging in nonshivering thermogenesis. While previously not thought to exist in adult humans, recent positron emission tomography scanning studies have demonstrated the existence of thermogenically active subscapular, mediastinal, and cervical BAT depots in adult humans that are induced by cold stress and beta-adrenergic stimuli [56]. Increased BAT mass is associated with metabolic health in humans, while decreased BAT mass is seen in aging, obesity, and metabolic disease. Finally, beige, or “brite”/brown-in-white adipocytes, reside in white adipose tissues, with a brown-like beneficial thermogenic metabolic phenotype induced by sympathetic stimuli, suggesting remarkable plasticity of adipocyte pheno-type. White and beige adipocytes appear to share a common preadipocyte stem cell, while brown adipocytes arise from a distinct stem cell precursor shared with myocytes [57]. Delineating the ontogeny of these different adipocyte phenotypes and identifying pathogenic and metabolically beneficial preadipocyte and adipocyte subpopulations is an active area of research.
Engineering adipose tissue
Early adipose tissue-targeted therapy focused on lipectomy. Not surprisingly given depot-specific disease associations, subcutaneous lipectomy in diabetic patients does not ameliorate diabetes [58]. Furthermore, while visceral lipectomy in mice improves systemic insulin resistance, similar interventions in humans, primarily in the form of omentectomy as an adjunct to bariatric surgery, are ineffective [59,60]. The discordance between murine and human visceral lipectomy data is likely multifactorial. Mice harbor the majority of VAT in an epididymal fat pad, resection of which is technically feasible. Human VAT in contrast consists of multiple subdepots, of which the omentum, the only subdepot amenable to resection, constitutes a smaller fraction; as such, omentectomy does not accomplish the same magnitude of visceral lipectomy as resection of the epididymal fat pad. Qualitative functional differences between murine and human adipose tissues also contribute, as the murine epididymal fat pad is functionally distinct from the human omentum. Finally, lipectomy is invariably followed by adipose tissue regeneration, which is rapidly induced by the hypothalamic adipostat after resection, limiting long-term systemic effects. Recent efforts focus instead on adipocyte plasticity. Pharmacologic “browning/beiging” strategies to shift white adipocytes toward brown/beige phenotypes are in various stages of preclinical research and clinical trials, including β3-adrenergic agents, PPAR-γ (peroxisome proliferator-activated receptor- γ) agonists, cGMP (cyclic guanosine monophosphate) induction agents, and fibroblast growth factor agonists [61]. Of interest, SAT is more susceptible to beiging than VAT, and this plasticity is decreased in obese humans, a potential explanation for endogenous SAT’s favorable influence on metabolism and its impairment in obesity [62].
Transplant of intact SAT or BAT into obese mice has beneficial metabolic effects [63], observations that form the basis of efforts to develop ex vivo engineered “designer” adipose tissue as a therapeutic vehicle [64]. Interestingly, such transplants only improve systemic metabolism when delivered into the visceral, but not subcutaneous, compartment [63]. This observation suggests that the peritoneal cavity is a preferred site for influencing systemic metabolism, possibly due to greater access to the portal venous circulation, while others demonstrate that transplanted tissue may undergo greater innervation in the visceral compartment [65]. Strategies include transplant of adipocytes embedded in biodegradable hydrogel matrices engineered to express browning agents to promote a BAT phenotype [64].
Anti-inflammatory drugs, such as salicylates and nonsteriodal anti-inflammatory drugs, have been studied as therapy for diabetes for decades. Research is ongoing into more specific agents, including selective cyclooxygenase inhibitors and monoclonal antibodies directed toward inflammatory cytokines, such as interluekin-1 and tumor necrosis factor-α [66]. Inhibitors of dipeptidyl peptidase-4, an enzyme expressed by adipose tissue that degrades the incretin hormone glucagon-like peptide 1, are currently in clinical use for diabetes, and recent research demonstrates that in addition to promoting pancreatic insulin secretion, these agents inhibit inflammation in adipose and other tissues [67], reinforcing the link between metabolism and immunity.
Conclusion
Multiple other avenues of research are directed toward developing adipose tissue-based therapy for metabolic disease. While leptin resistance has so far limited leptin’s therapeutic utility, recent data demonstrate that pharmacologic agents that reduce hypothalamic endoplasmic reticulum stress may ameliorate leptin resistance, rendering leptin therapy for obesity efficacious [68]. These findings promise realization of the hope that the discovery of leptin provided for the obesity epidemic over 2 decades ago.
Supplementary Material
Acknowledgments
R.W.O. is supported by National Institutes of Health Grants R01 DK097449, R01 DK115190, Veterans Administration Merit Grant I01 CX001811, and a Pilot and Feasibility Grant from the Michigan Diabetes Research Center (NIH Grant P30-DK020572).
Footnotes
Disclosures
The authors have no commercial associations that might be a conflict of interest in relation to this article.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.soard. 2018.07.032.
References
- [1].Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, friedman JM. Positional cloning of the mouse obese gene and its human homo-logue. Nature 1994;372(6505):425–32. [DOI] [PubMed] [Google Scholar]
- [2].Farooqi IS, Jebb SA, Langmack G, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 1999;341(12):879–84. [DOI] [PubMed] [Google Scholar]
- [3].Considine RV, Sinha MK, Heiman ML, et al. Serum immunore-active-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996;334(5):292–5. [DOI] [PubMed] [Google Scholar]
- [4].Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012;307(5):491–7. [DOI] [PubMed] [Google Scholar]
- [5].Lewis CE, McTigue KM, Burke LE, et al. Mortality, health outcomes, and body mass index in the overweight range: a science advisory from the American Heart Association. Circulation 2009;119(25):3263–71. [DOI] [PubMed] [Google Scholar]
- [6].Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief 2015;219:1–8. [PubMed] [Google Scholar]
- [7].American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36(4):1033–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stokes A, Ni Y, Preston SH. Prevalence and trends in lifetime obesity in the U.S. 1988–2014. Am J Prev Med 2017;53(5):567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 2010;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Finkelstein EA, Brown DS, Wrage LA, Allaire BT. Hoerger TJ Individual and aggregate years-of-life-lost associated with overweight and obesity. Obesity (Silver Spring) 2010;18(2):333–9. [DOI] [PubMed] [Google Scholar]
- [11].Livi R L’indice ponderale o rapport0 tra la statura e il peso. Atti Sot Romana Antrop 1897;5:125–53. [Google Scholar]
- [12].Quetelet A Recherches sur le poids de l’homme aux differens ages. Nouv Mem Acad Roy Sci Belleslett Bruxelles 1832;7:1–43. [Google Scholar]
- [13].Quetelet A Physique sociale ou Essai sur le developpement de ses facultes de l’homme, Vol 2. Bruxelles: Mucquardt; 1869. [Google Scholar]
- [14].Eknoyan G Adolphe Quetelet (1796–1874)-the average man and indices of obesity. Nephrol Dial Transplant 2008;23(1):47–51. [DOI] [PubMed] [Google Scholar]
- [15].Dublin LI, Lotha AJ. Twenty-five years of health progress: A study of the mortality experience among the industrial policyholders of the metropolitan life insurance company, 1911 to 1935. New York, NY, USA: Metropolitan Life Insurance; 1937. [Google Scholar]
- [16].Medico-Actuarial Mortality Investigation, Vol.1. New York: Association of Life Insurance Medical Directors and the Actuarial Society of America; 1912. https://archive.org/details/cu31924104606193. [PubMed] [Google Scholar]
- [17].Florey CV. The use and interpretation of ponderal index and other weight-height ratios in epidemiological studies. J Chronic Dis 1970;23(2):93–103. [DOI] [PubMed] [Google Scholar]
- [18].Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and adiposity. J Chronic Dis 1972;25(6):329–43. [DOI] [PubMed] [Google Scholar]
- [19].World Health Organization. Physical status: the use and interpretation of anthropometry. Geneva, Switzerland: Technical Report Series, No. 854; 1995. [Google Scholar]
- [20].Berrington de Gonzalez A Hartge P Cerhan JR et al. Body-mass index and mortality among 1.46 million white adults. N Eng J Med 2010;363(23):2211–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Flegal KM, Kit BK, Orpana H, Graubard BI, Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013;309(1):71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW Jr. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med 1999;341(15):1097–105. [DOI] [PubMed] [Google Scholar]
- [23].Di Angelantonio E, Bhupathiraju ShN, Wormser D, et al. Body-mass index and all-cause mortality: individual-participant data meta-analysis of 239 prospective studies in four continents. Lancet 2016;388(10046):776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Di Angelantonio E, Bhupatiraju ShN, et al. , The Global BMI Mortality Collaboration Body-mass index and all-cause mortality: individual participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016;388(10046):776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].The GBD 2015 Obesity Collaborators Health effects of over-weight and obesity in 195 countries over 25 years. N Eng J Med 2017;37(1):13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Burkhauser RV, Cawley J. Beyond BMI: the value of more accurate measures of fatness and obesity in social science research. J Health Econ 2008;27(2):519–29. [DOI] [PubMed] [Google Scholar]
- [27].Kanazawa M, Yoshiike N, Osaka T, Numba Y, Zimmet P, Inoue S. Criteria and classification of obesity in Japan and Asia-Oceania. World Rev Nutr Dietetics 2005;94:1–12. [DOI] [PubMed] [Google Scholar]
- [28].Who Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363(9403):157–63. [DOI] [PubMed] [Google Scholar]
- [29].Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med 2008;168(15):1617–24. [DOI] [PubMed] [Google Scholar]
- [30].Rubino F, Nathan DM, Eckel RH for the Delegates of the 2nd Diabetes Surgery Summit. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: a joint statement by International Diabetes Organizations. Diabetes Care 2016;39(6):861–77. [DOI] [PubMed] [Google Scholar]
- [31].Blüher M The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr Opin Lipidol 2010;21(1):38–43. [DOI] [PubMed] [Google Scholar]
- [32].Stefan N, Schick F, Haring HU. Causes, characteristics, and consequences of metabolically unhealthy normal weight in humans. Cell Met 2017;26(2):292–300. [DOI] [PubMed] [Google Scholar]
- [33].Gujral UP, Vittinghoff E, Mongraw-Chaffin M. et al. Cardiometabolic abnormalities among normal-weight persons from five racial/ethnic groups in the United States: a cross-sectional analysis of two cohort studies. Ann Intern Med 2017;166(9):628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Colman E Dinitrophenol and obesity: an early twentieth-century regulatory dilemma. Regul Toxicol Pharmacol 2007;48(2):115–17. [DOI] [PubMed] [Google Scholar]
- [35].Tainter ML, Stockton AB, Cutting WC. Use of dinitrophenol in obesity and related conditions: a progress report. JAMA 1933;101(19):1472–5. [Google Scholar]
- [36].Smith CE, Ordovas JM. Update on perilipin polymorphisms and obesity. Nutr Rev 2012;70(10):611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Johannsen DL, Tchoukalova Y, Tam C, et al. Effect of 8 weeks of overfeeding on ectopic fat deposition and insulin sensitivity: testing the adipose tissue expandability hypothesis. Diabetes Care 2014;37(10):2789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Muir LA, Neeley CK, Meyer KA, et al. Adipose tissue fibrosis, hypertrophy, and hyperplasia: correlations with diabetes in human obesity. Obesity (Silver Spring) 2016;24(3):597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hotamisligil GS, Davis RJ. Cell signaling and stress responses. Cold Spring Harb Perspect Biol 2016;8(10):a006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Segerstrom SC. Stress, energy, and immunity: an ecological view. Curr Dir Psychol Sci 2007;16(6):326–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li C, Xu MM, Wang K, et al. Macrophage polarization and meta-inflammation. Transl Res 2018;191:29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Divoux A, Tordjman J, le Lacasa D, et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 2010;59(11):2817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mehta R, Jeiran K, Koenig AB, et al. The role of mitochondrial genomics in patients with non-alcoholic steatohepatitis (NASH). BMC Med Genet 2016;17(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].DeFronzo RA, Ferrannini E, Sato Y, Felig P, Wahren J. Synergistic interaction between exercise and insulin on peripheral glucose uptake. J Clin Invest 1981;68(6):1468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG. Quantification of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13 C nuclear magnetic resonance spectroscopy. N Engl J Med 1990;322(4):223–8. [DOI] [PubMed] [Google Scholar]
- [46].Klein S, Allison DB, Heymsfield SB the American Diabetes Association. Waist circumference and cardiometabolic risk: a consensus statement from shaping America’s health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Diabetes Care 2007;30(6):1647–52. [DOI] [PubMed] [Google Scholar]
- [47].Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007;116(1):39–48. [DOI] [PubMed] [Google Scholar]
- [48].Bouchi R, Takeuchi T, Akihisa M, et al. High visceral fat with low subcutaneous fat accumulation as a determinant of atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol 2015;14:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Verboven K, Wouters K, Gaens K, et al. Abdominal subcutaneous and visceral adipocyte size, lipolysis and inflammation relate to insulin resistance in male obese humans. Sci Rep 2018;8(1):4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Fox CS, Pencina MJ, Meigs JB, Vasan RS, Levitzky YS, D’Agostino RB Sr. Trends in the incidence of type 2 diabetes mellitus from the 1970s to the 1990s: the Framingham Heart Study. Circulation 2006;113(25):2914–18. [DOI] [PubMed] [Google Scholar]
- [51].Shadid S, Koutsari C, Jensen MD. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes 2007;56(5):1369–75. [DOI] [PubMed] [Google Scholar]
- [52].Fabbrini E, Yoshino J, Yoshino M, et al. Metabolically normal obese people are protected from adverse effects following weight gain. J Clin Invest 2015;125(2):787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lee T-WA, Kwon H, Zong H, et al. Fyn deficiency promotes a preferential increase in subcutaneous adipose tissue mass and decreased visceral adipose tissue inflammation. Diabetes 2013;62(5):1537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Barreira TV, Harrington DM, Staiano AE, Heymsfield SB, Katz-marzyk PT. Body adiposity index, body mass index, and body fat in white and black adults. JAMA 2011;306(8):828–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Friedl KE, Moore RJ, Martinez-Lopez LE, et al. Lower limit of body fat in healthy active men. J Appl Physiol 1994;77(2):933–40. [DOI] [PubMed] [Google Scholar]
- [56].van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009;360(15):1500–8. [DOI] [PubMed] [Google Scholar]
- [57].Wu J, Boström P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012;150(2):366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Klein S, Fontana L, Young VL, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med 2004;350(25):2549–57. [DOI] [PubMed] [Google Scholar]
- [59].Dunn JP, Abumrad NN, Breitman I, et al. Hepatic and peripheral insulin sensitivity and diabetes remission at 1 month after Roux-en-Y gastric bypass surgery in patients randomized to omentectomy. Diabetes Care 2012;35(1):137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Shi H, Strader AD, Woods SC, Seeley RJ. The effect of fat removal on glucose tolerance is depot specific in male and female mice. Am J Physiol Endocrinol Metab 2007;293(4):E1012–20. [DOI] [PubMed] [Google Scholar]
- [61].Abdullahi A, Jeschke MG. Taming the flames: targeting white adi-pose tissue browning in hypermetabolic conditions. Endocr Rev 2017;38(6):538–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Seale P, Conroe HM, Estall J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Investig 2011;121(1):96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab 2008;7(5):410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tharp KM, Stahl A. Bioengineering beige adipose tissue therapeutics. Front Endocrinol (Lausanne) 2015;6:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Stanford KI, Middelbeek RJ, Townsend KL, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 2013;123(1):215–23, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Goldfine AB, Shoelson SE. Therapeutic approaches targeting inflammation for diabetes and associated cardiovascular risk. J Clin Invest 2017;127(1):83–93, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Bae EJ. DPP-4 inhibitors in diabetic complications: role of DPP-4 beyond glucose control. Arch Pharm Res 2016;39(8):1114–28, [DOI] [PubMed] [Google Scholar]
- [68].Ozcan L, Ergin AS, Lu A, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab 2009;9(1):35–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.