Abstract
Very little research has assessed how measures of negative and positive affect (NA and PA) derived from assessments at multiple time points per day (e.g., via ecological momentary assessment [EMA]), as opposed to questionnaires that rely on recall over a longer period, are related to levels of peripheral inflammation. We examined how different indicators of NA and PA predicted concentrations of C-reactive protein (CRP) and seven peripheral inflammatory cytokines (IL-1β, IL-6, TNF-α, IL-8, IL-4, IL-10, and IFN-γ) that were examined in the form of an inflammatory composite. A community-based sample of 220 adults (62% Black/African-American and 25% Hispanic/Latino; aged 25–65; 65% female) completed questionnaires at baseline (including recalled affect “over the past month”) and then provided EMA reports 5x/day for 14 days. Blood was drawn from each participant after completion of EMA and used to determine plasma levels of CRP and cytokines. Analyses examined if indicators of affect predicted inflammation, controlling for age, gender, body mass index, education, health conditions, and statin use. Neither recalled NA or PA nor momentary NA or PA (aggregated across the 14 days of EMA) were significantly associated with the cytokine composite or CRP. Negative mood more proximal to the blood draw (i.e., aggregated momentary NA in week 2 of EMA) was associated with the cytokine composite but not CRP. Exploratory moderation analyses revealed that the cytokine composite was also associated with PA in week 2 for men only, and with recalled NA for those with lower education. Exploratory analyses around temporal dynamics suggested that the timing of NA measurement relative to the blood draw mattered: Specifically, there were stronger trends of association between momentary NA and inflammatory cytokines when NA was assessed closer in time to blood collection. Future investigation of the relevance of temporal proximity and other measurement details may improve understanding of how affect relates to inflammation.
Keywords: Emotion, Mood, Negative Affect, Positive Affect, Inflammation, Cytokine, CRP, Ecological Momentary Assessment
1. Introduction
Although it is well established that peripheral inflammation is related to individual differences in constructs that involve affect, such as depression and hostility (Grahamet al., 2006; Hackett, Lazzarino, Carvalho, Hamer, & Steptoe, 2015; Irwin, 2001; Marsland, Prather, Petersen, Cohen, & Manuck, 2008; Miller & Raison, 2016; Miller, Freedland, Carney, Stetler, & Banks, 2003; Suarez, Lewis, Krishnan, & Young, 2004), it remains less clear how inflammation is specifically related to measures of negative and positive affect (NA and PA). Greater understanding of the relationship between affect and inflammation is important because it may help identify modifiable pathways by which psychosocial factors predict chronic inflammation, a risk factor for multiple diseases, frailty, and mortality (Ferrucciet al., 1999; Pradhan, Manson, Rifai, Buring, & Ridker, 2001; Stoneret al., 2013). Some recent studies have focused on the association of inflammatory biomarkers with recalled affect (from standard questionnaire measures where participants retrospectively report on their affect over the past weeks or month) and at least one study has used momentary experiences of affect (derived from ecological momentary assessment [EMA]); findings have been inconsistent or limited in various ways, as reviewed in greater detail below (e.g., Andreassonet al., 2013; Carrollet al., 2011; Steptoe, O’Donnell, Badrick, Kumari, & Marmot, 2008; Sturgeonet al., 2016). Many nuances with regard to how affect and inflammation are related remain unexplored. To our knowledge, no one has examined the degree to which assessment methods or timing modify the association between affect and inflammation. The primary goal of the present research was to examine linkages between NA and PA with biomarkers of inflammation in a diverse sample of adults, using affect ratings obtained from both standard recall and EMA, and to explore whether the temporal proximity between measures of momentary affect and inflammation influences these relationships.
Measurement differences between studies may help explain inconsistencies in the emerging literature linking affect and inflammation. Recalled measures of affect (e.g., how often in the last month did you feel sad?) are commonly used and can offer valuable information about how a person views themselves or their past experience; however, they are susceptible to memory bias, and ratings on these scales appear to be influenced by personality and self-beliefs (Shiffman, Stone, & Hufford, 2008). In contrast, measures of momentary affect (e.g., how much do you feel sad right now?) may better capture actual affective experiences in real-time; although they are still self-reported and can reflect desirability bias, such measures minimize recall bias (Schwarz, 2007; Smyth & Heron, 2012). A growing body of evidence indicates that momentary assessments are, in some cases, better predictors of health and physiological responses (e.g., longevity, cortisol awakening response, blood pressure) than are recalled measures (Bajajet al., 2016; Cohen, Alper, Doyle, Treanor, & Turner, 2006; Conner & Barrett, 2012; Daly, 2012; Kamarcket al., 2005; Steptoe, Gibson, Hamer, & Wardle, 2007; Stoneet al., 1994). For example, momentary measures of psychosocial stress, including negative affect, were related to cardiovascular measures (e.g., ambulatory blood pressure, heart rate) whereas comparable global measures of stress were not (Kamarck et al., 2005). In another study, both global recalled PA and aggregated momentary PA predicted lower systolic blood pressure throughout laboratory stress tasks, yet only aggregated momentary PA was linked to faster post-stress diastolic blood pressure recovery (Steptoe et al., 2007). Sometimes, however, recalled self-assessments are stronger predictors of health outcomes, perhaps because they tap into trait-like tendencies or important beliefs about the self (Kahneman & Riis, 2005; Robinson & Clore, 2002). To our knowledge, no one has examined associations between inflammation and affect measures derived from EMA as well as global recall within the same sample of participants.
Only one study of which we are aware has examined a linkage between EMA-derived affect (where individuals were asked how they felt in the moment at multiple time points across the day) and peripheral inflammation. Among nearly 2,900 midlife and older British adults, women with higher average momentary PA over the course of a day had lower C-reactive protein (CRP) and interleukin (IL)-6 (as determined from a blood draw the day before) compared to women with lower average PA. The associations were independent of depressive symptoms, but NA was not reported in this study. No such associations were observed among men (Steptoe et al., 2008).
There is relatively more research on the associations of global recalled NA with peripheral inflammation but this literature has produced mixed findings. In a sample of middle-age community dwelling adults, recalled NA over the past 7 days was related to higher IL-6 but not CRP, as determined from plasma obtained the same day as questionnaire assessment (Sturgeon et al., 2016). Another study found an association between recalled NA over the past 30 days and greater IL-6 in a sample from the U.S. but not one from Japan (Miyamotoet al., 2013), using blood samples from an indeterminate period of time from questionnaire assessment. In a recent analysis from a national study of U.S. adults aged 25–34, there was a non-significant trend for NA from the past week to be associated with CRP derived from blood spots obtained immediately following questionnaire assessment (Blevins, Sagui, & Bennett, 2017). Similarly, among 347 Swedish women aged 45–90, mostly null findings were reported between recalled NA over the past two weeks and proinflammatory cytokines (e.g., IL-6, IL-1β) determined from plasma obtained the same day as questionnaire assessment, with the exception of a correlation between NA and soluble IL-2 receptor (sIL-2r) (Andreasson et al., 2013).
The literature linking recalled PA and peripheral inflammation is also varied, with a predominance of null findings. Recalled PA over the past week has been linked with lower IL-6 determined from plasma the same day in healthy adults (Sturgeon et al., 2016). However, recalled PA over the past two weeks was not significantly associated with cytokine levels (e.g., IL-1β, IL-6) determined from plasma obtained the same day in a sample of Swedish women (Andreasson et al., 2013). Recalled PA from the past week was not significantly associated with two inflammatory markers (IL-6, sIL-6r) determined from a blood sample approximately one month post-questionnaire assessment among a sample of older women (Friedman, Hayney, Love, Singer, & Ryff, 2007). Further, among adults aged 25–34, recalled PA from the past week was not significantly associated with CRP derived from blood spots obtained immediately after questionnaire assessment (Blevins et al., 2017).
In sum, the literature linking recalled global affect with inflammation is inconsistent with regard to findings, and the literature linking momentary affect with inflammation is scant (one study). A number of factors may help explain these inconsistencies, such as diverse assessment techniques (e.g., blood sampling by blood spot versus blood draw; different measures of affect), sample characteristics, and the timing between affect and inflammatory assessment. Further, the bulk of past research related to a connection between affect and inflammation has utilized a limited number of inflammatory markers (usually one or two markers in a given study) examined in isolation. To our knowledge the association between momentary affect and inflammation has not been investigated with a broad panel of markers that includes both pro-and anti-inflammatory cytokines.
The first goal of the present research was to examine recalled as well as momentary affect in relation to peripheral inflammation in middle-aged adults. In the present study, recalled affect ratings were obtained at baseline, followed by EMAs of affect 5 times per day for 14 days, concluding with a blood draw. For our key inflammatory measures, we examined CRP as well as a composite measure of inflammatory cytokines that included both pro-and anti-inflammatory markers. We hypothesized that both recalled and momentary affect would be associated with inflammation, with NA measures associated with higher levels and PA measures associated with lower levels. Given that affect and inflammatory states can fluctuate in response to numerous factors (e.g., Montpetit, Bergeman, Deboeck, Tiberio, & Boker, 2010; Steptoe, Hamer, & Chida, 2007), we also reasoned that associations between momentary affect and inflammation may be more apparent when they are assessed closer in time. Thus, a second goal was to contrast associations of momentary affect ratings obtained during the week proximal to the blood draw with the same measures obtained during the week further from the blood draw, with the expectation that the former would be more predictive of inflammation. On an exploratory basis, we examined moderation of primary findings by race, ethnicity, age, education, and gender given past literature linking all of these important sociodemographic factors with health disparities and with inflammatory markers specifically (e.g., Darnall & Suarez, 2009; Graham, Christian, & Kiecolt-Glaser, 2006; O’Connoret al., 2009; Ransome, Slopen, Karlsson, & Williams, 2018). In particular, several studies have observed interactions by gender in the connection between affect and inflammation (e.g., Sin, Graham-Engeland, Ong, & Almeida, 2015; Steptoe et al., 2008).
2. Methods
2.1. Participants
Data came from an ongoing study called the “Effects of Stress on Cognitive Aging, Physiology, and Emotions” (ESCAPE) Study. ESCAPE employs a longitudinal design with four waves of data collection, spaced 9–12 months apart (Scottet al., 2015). The current analysis used data from the first (baseline) wave only, as data collection is still underway. Participants were recruited from Co-op City, a cooperative housing development designed to provide affordable housing to residents in the Bronx, NY, and the surrounding area. To be eligible for the study, participants had to be aged 25–65 years, fluent in English, and not visually impaired. Of the 240 total participants recruited in the first wave of the ESCAPE protocol (Scott et al., 2015) who were offered the opportunity of having their blood drawn, 226 provided blood samples at the end of the EMA phase. The 14 participants who dropped out or did not have their blood drawn (for a variety of reasons, including schedule issues, illness, or difficulty with the blood draw) did not differ from the final analytic sample on any demographic characteristic examined in the present research. Three participants were excluded from analyses because they were taking immunosuppressive drugs (e.g., corticosteroids); an additional 3 participants were missing body mass index (BMI). The final analytic sample of 220 was 64% female with a mean age of 46.21 (SD = 11.12). Additional participant characteristics are described below.
2.2. Procedures
The EMA data collection period started with an in-person visit during which a research technician instructed participants on how to use a customized smartphone for the EMA assessments. A 2-day practice phase followed, during which participants who were unable to achieve at least 80% completion of the EMA assessments were excluded from further participation (for details, see Scott et al., 2015). Following this practice phase, participants completed 14 consecutive days of EMA when prompted by phone “beeps” 5 times per day, which were distributed in stratified intervals that covered the waking day of participants with the constraint that that “beeps” could not occur within 30min of each other. As soon as possible after this EMA data collection period (median = 3 days later; mode = 1, SD = 4.6, range = 1–21 days later), participants were asked to visit the Einstein Medical Center to return the smartphone and provide a blood sample. Although the majority (>86%) did so within a week, some were delayed due to factors such as travel, family commitments, acute illness, and other schedule-related issues. Our primary analyses described below were also run separately after excluding individuals who returned to the medical center more than one week after the end of their last EMA assessment; results did not differ meaningfully with these individuals removed.
2.3. Measures
2.3.1. Inflammatory biomarkers.
The blood draw was performed by a certified phlebotomist and took place between 7:00am and 11:00am, following 12 hours of fasting. Participants sat for 20min prior to the blood draw. The blood samples used in the present research were collected in sodium heparin coated tubes for cytokine analysis and EDTA coated tubes for CRP analysis. Heparin samples were maintained at room temperature and EDTA samples were kept on ice prior to centrifugation (3000g x 15 min). The supernatant was stored at −80°C; every six months aliquots were shipped overnight on dry ice to the Stress and Immunity Laboratory at Penn State University for analysis.
High sensitivity CRP was determined from blood plasma using ELISA (Cayman Chemical, Ann Arbor MI), and a set of inflammatory cytokines was determined from blood plasma using multiplex bead arrays (Thermo Fisher Scientific, Waltham, MA). For CRP, the minimum detection limit was 46.9 pg/mL, the intra-assay coefficients of variation (CVs) ranged from 1.9–7.0%, and the inter-assay CV was 9.84%. For cytokines, the minimum detection limit ranged from 0.02–2.77 pg/mL, and the inter-assay CVs ranged from 7.0–9.8%. All assays were performed in duplicate. For the current analyses, we selected specific inflammatory biomarkers a priori that have been linked to stress, NA, and/or PA in past research: CRP as a broad marker of systemic inflammation; the classic proinflammatory cytokines IL-1β, IL-6, TNF-α; the anti-inflammatory cytokines IL-4, IL-10; a chemokine for neutrophils, IL-8; and interferon-γ (IFN-γ), which plays an important role during infection in innate immunity by activating macrophages. The process by which these cytokines were aggregated is described below.
2.3.2. Recalled affect.
Recalled PA and NA were assessed at baseline with an adjective checklist scale that included select items from the Positive and Negative Affect Schedule (PANAS; Watson et al., 1988) as well as other items. Participants were asked how often they felt each emotion listed over the past month on a scale from 1 (not at all) to 7 (extremely). PA was assessed with 10 items: happy, alert, enthusiastic, excited, cheerful, relaxed, content, peaceful, calm, and satisfied. NA was assessed with 10 items: irritable, sad, tense, bored, stressed, depressed, nervous, sluggish, upset, and disappointed. NA and PA subscales had excellent internal consistency reliability (Cronbach’s alpha = .90 and .94, respectively).
2.3.3. Momentary affect.
During the EMA data collection phase, when prompted by the smartphones, participants rated the extent to which they currently felt four PA items (happy, pleased, enjoyment/fun, joyful) and five NA items (tense/anxious, angry/hostile, depressed/blue, frustrated, unhappy). Ratings were made on a continuous sliding scale from “not at all” to “extremely” (this equated to a scale from 0–100; numeric ratings were not visible to participants). Internal consistency was calculated according to procedures described by Cranford and colleagues (2006). PA and NA demonstrated good internal consistency reliability (internal consistency = 0.93 for PA and 0.93 for NA). Items were averaged within each subscale to obtain momentary ratings of positive and negative affect; momentary affect scores were then aggregated across all EMAs (up to 70 momentary assessments; i.e., 5x/day for 14 days). For analyses comparing momentary affect by week, momentary affect was averaged separately for days 1–7 of the EMA protocol (i.e., Week 1, which occurred further in time from the blood draw) and for days 8–14 (i.e., Week 2, which was closer in time to the blood draw). The reliabilities of the momentary affect measures were similar across weeks 1 and 2 (internal consistency of .87 for PA and NA in Week 1 and Week 2).
2.4. Covariates
To better determine the unique associations of NA and PA with inflammation, we controlled for gender, age, and BMI because all are commonly related to inflammation (for review see O’Connor et al., 2009). We also controlled for the number of chronic conditions reported (from a list of 26 conditions, e.g., high blood pressure, kidney problems) and for statin use. As noted earlier, the few ESCAPE participants taking anti-inflammatory medications such as prednisone were not included in the present analyses. For a measure of socioeconomic status, we controlled for education instead of income because ~10% of participants did not provide income data. Further, education was strongly associated with income [X2(1, 212) = 15.11, p< .0001] and, unlike income, was associated with both inflammatory variables in the present research (details in section 3.1). Finally, we considered the sociodemographic and behavioral factors of marital status, race, ethnicity, and tobacco use as additional potential covariates on the basis of whether they were associated with inflammatory markers; based on these analyses (details in section 3.1), none of these variables were included as additional covariates.
2.5. Statistical analyses
Statistical analyses were performed with SAS 9.4 (SAS Institute, Cary, NC). Inflammatory biomarkers were log-10 transformed prior to analyses to correct for non-normal distribution; for all biomarkers, a log (x+1) formula was applied because many values were less than one (Tabachnick & Fidell, 2001). To utilize data from the maximum number of participants while minimizing the influence of outliers on all analyses, outliers greater than 3 SD above the mean were winsorized to 3 SD for each cytokine (i.e., replaced with the appropriate value based on the distribution) based on precedent (Out, Hall, Granger, Page, & Woods, 2012) and statistical recommendations (Tabachnick & Fidell, 2001); there were no outliers greater than 3 SD above the mean for CRP.
We examined whether the seven cytokines of interest (IL-1β, IL-4, IL-6, IL-8, IL-10, TNF-α, and IFN-γ) and CRP could be grouped into a composite to represent overall inflammatory load. All cytokines were positively associated with one another (r’s ranging from 0.41 to 0.89). Principal components analysis with varimax rotation showed that all of the cytokines formed a single factor (factor loadings for the individual cytokines ranged from 0.74 to 0.92), with CRP most appropriately considered a separate factor; details of this factor analysis are shown in supplemental Table S1. Consistent with previous research (Dornet al., 2016; Pripp & Stanisic, 2014), anti-inflammatory cytokines were positively correlated with proinflammatory cytokines when examined in cross-section; these cytokines often correlate because anti-inflammatory cytokines rise to buffer (or limit) inflammation. To form a composite inflammation score, we first z-scored the seven log-transformed cytokines (to standardize the distribution of scores across these cytokines) and then averaged them. Separate linear regression models were run to determine the associations of recalled and momentary affect with the cytokine composite and CRP. We focused on the cytokine composite to minimize Type 1 error due to multiple comparisons. However, because individual cytokines may be of particular interest to readers, results of regression analyses to predict individual cytokines are presented in supplemental tables (Tables S2–3). Continuous predictors and covariates were standardized (z-scored) so that results can be interpreted as standardized betas, whereas betas for dummy coded covariates can be interpreted as differences between groups.
For moderation analyses, we used multiple regression to examine whether key demographic variables (age, gender, race, ethnicity, and education) moderated our primary analysis linking affect (both recalled and momentary) with the cytokine composite and CRP (across weeks and by Week 1 and Week 2). Moderation by age was examined using age as a continuous variable; all other moderators were dummy coded variables. Moderation analyses by race combined those identifying as either non-Hispanic/Black or Hispanic/Black and compared them to those identifying with all other racial groups. Analyses by ethnicity compared Hispanic participants (identifying with either White or Black race) with non-Hispanic participants. Any significant interaction term (p < .05) was further tested with simple slopes analyses to determine the directions of the observed associations.
3. Results
3.1. Participant characteristics and preliminary findings
Detailed participant characteristics are shown in Table 1. The analytic sample was racially and ethnically diverse (62% Black/African American, 25% Hispanic/Latino) as well as socioeconomically diverse (median household income of $40–50,000 with 45% having completed college education or higher). Median BMI was 30.78 (mean = 32.01, SD = 8.31), indicating that the average participant was obese. Health status was also diverse, as indicated by number of chronic conditions and a wide range of CRP levels with a relatively high average CRP (mean = 6.17 mg/L, SD =9.40); 67 participants had CRP levels greater than 3mg/L, a level considered to confer some risk of cardiovascular disease (Conen & Ridker, 2007).
Table 1.
Sample demographics and characteristics (N = 220)
| Characteristics | Mean (SD) or N (%) |
|---|---|
| Age, years | 46.21 (11.12) |
| Female | 140 (64.94%) |
| Body mass index | 32.05 (8.28) |
| African-American / Black (non-Hispanic) | 62.34% |
| Hispanic Black | 6.3% |
| Hispanic White | 17.7% |
| White (non-Hispanic) | 8.23% |
| Other race (including Asian, American Indian, Alaska Native) |
4.33% |
| Married/Cohabiting | 94 (40.87%) |
| Household income < $40,000 | 99 (43.23%) |
| College graduate | 105 (45.45%) |
| Number of chronic conditions | 3.33 (2.57) |
| Statin use | 22 (9.52%) |
| CRP, mg/L | 6.17 (9.40) |
Higher education (college degree) was significantly associated with lower levels of the cytokine composite and CRP (see Table 2); for this reason, education was included as a covariate and as an important indicator of socioeconomic status as described earlier. Race, ethnicity, marital status, and tobacco use were not significantly associated with any inflammatory marker and thus were not included in the models. The final set of covariates in key analyses was thus age, gender, BMI, education, health conditions, and statin use. Associations between participant characteristics and inflammation variables are shown in Table 2.
Table 2.
Bivariate associations between key participant characteristics and inflammation
| Participant characteristic |
Cytokine composite |
CRP |
|---|---|---|
| Body mass index | r = −0.08 | r = 0.53*** |
| Chronic conditions | r = −0.03 | r = 0.15* |
| Statin use | t = −1.09s | t = 1.01 |
| Tobacco use | t = −1.34 | t = −1.21 |
| Age | r = 0.08 | r = −0.03 |
| Male gender | t = −1.98*s | t = −0.42 |
| African-American/Black | t = 0.66 | t = 0.93 |
| Hispanic | t = −1.03 | t = −0.14 |
| College graduate | t = −1.69†s | t = −3.11** |
| Married | t = −1.40 | t = 0.38 |
Note. Pearson correlations were computed for continuous variables and t-tests were used for dichotomous variables. All analyses were performed on log-10 transformed biomarkers. Participants were coded as married if they were either married or habitating as if married. Correlations indicated that greater body mass index and number of chronic conditions were associated with higher CRP. T-tests suggested that males had higher levels of the cytokine composite than females, and that individuals with higher education than others had lower CRP.
indicates equal variance could not be assumed and Satterthwaite estimation for unequal variance was used.
p ≤ 0.001
p ≤ 0.01
p ≤ 0.05
p ≤ 0.10
The number of total “beeps” with EMA data was a mean of 55.5 (SD = 13.8; median = 60; mode = 68) out of a total of 70 (for an overall compliance rate of ~80%, similar to other EMA studies using similar sampling densities and durations). The number of days with at least one EMA report provided was a mean of 13.23 days (SD = 1.60; median = 14, mode = 14) out of a total of 14 (~95% days with at least some EMA data); Week 2 compliance was slightly lower than Week 1 but still high (e.g., 97% of days with EMAs in Week 1 versus 94% in Week 2).
Average levels of recalled NA and PA, aggregated momentary NA and PA, and their bivariate associations are shown in Table 3. As expected, recalled NA and PA were negatively correlated, as were measures of momentary NA and PA. Recalled and average momentary affect were only moderately correlated (r = 0.33 for NA and r = 0.53 for PA).
Table 3.
Affect measures
| Pearson r correlations | |||||
|---|---|---|---|---|---|
| Affect measure | Mean (SD) or N (%) |
Recalled NA |
Recalled PA |
Momentary NA |
Momentary PA |
| Recalled | 35.42 (12.67) | ---- | |||
| Recalled PA | 45.21 (12.12) | −0.49*** | ---- | ||
| Momentary NA | 23.40 (16.06)76) | 0.32*** | −0.32*** | ---- | |
| Momentary PA | 60.65 (18.76) | −0.38*** | 0.54*** | −0.48*** | ---- |
p ≤ 0.001
3.2. Recalled affect and two-week aggregated momentary affect with inflammation
We first examined the degree to which recalled affect and aggregated momentary affect were associated with the cytokine composite and CRP; our expectation was that both recalled and momentary affect would relate to inflammatory biomarkers. Table 4 shows the results of regression analyses, which controlled for age, gender, BMI, education, health conditions, and statin use. Neither recalled NA or PA, nor momentary NA or PA (aggregated across the full 14 days) were significantly associated with the composite inflammatory cytokine measure or CRP (see Table S2 for associations with individual cytokines, which were mostly non-significant).
Table 4.
Recalled and momentary affect as predictors of inflammatory markers
| Affect Measure | Standardized ß (95% Confidence Interval) |
|
|---|---|---|
| 7-cytokine composite | CRP | |
| Recalled ΝΑ | −0.018 (−0.103, 0.066) | 0.001 (−0.035, 0.056) |
| Momentary ΝΑ | 0.051 (−0.032, 0.135) | 0.019 (−0.026, 0.063) |
| Recalled PA | 0.038 (−0.046, 0.121) | −0.011 (−0.056, 0.034) |
| Momentary PA | 0.001 (−0.004, 0.008) | −0.025 (−0.070, 0.020) |
Note. Momentary affect in these analyses was aggregated across the full two weeks of EMAs. Analyses controlled for age, gender, body mass index, education, number of chronic conditions, and statin use. Analyses were performed on log-10 transformed biomarkers. No significant associations were observed.
3.3. Momentary affect by week with inflammation
Next, we examined the degree to which momentary affect in week 1 and week 2 separately were associated with the cytokine composite and CRP, using the same set of covariates. We expected that week 2 momentary affect – which was measured closer in time to the blood draw – would be more strongly associated with inflammatory markers than week 1 momentary affect. As shown in Table 5, greater week 2 momentary NA was associated with higher levels of the cytokine composite but not CRP (see Table S3 for associations with individual cytokines). Week 2 momentary PA was not significantly associated with the cytokine composite or CRP. In week 1, there were no significant associations between momentary NA or PA with either the cytokine composite or with CRP. On an exploratory basis, to probe the robustness of the association between Week 2 NA and the cytokine composite we added recalled NA as a covariate; average momentary NA in Week 2 remained a significant predictor.
Table 5.
Momentary affect from Week 1 (further from blood draw) and Week 2 (proximal to blood draw) as predictors of inflammatory markers
| Affect Measure | Standardized ß (95% Confidence Interval) |
|
|---|---|---|
| 7-cytokine composite | CRP | |
| Week 1 ΝΑ | 0.017 (−0.067, 0.102) | 0.011 (−0.033, 0.056) |
| Week 2 ΝΑ | 0.087 (0.005, 0.170)* | 0.026 (−0.019, 0.070) |
| Week 1 PA | 0.032 (−0.054, 0.119) | −0.013 (−0.059, 0.032) |
| Week 2 PA | −0.037 (−0.122, 0.047) | −0.033 (−0.077, 0.012) |
Note. Analyses controlled for age, gender, body mass index, education, number of chronic conditions, and statin use. Analyses were performed on log-10 transformed biomarkers.
p ≤ 0.05
3.4. Temporal dynamics in the association between momentary affect and inflammation
On an exploratory basis, to further examine the role of temporal proximity, individual regression analyses were performed between momentary NA and the cytokine composite, with the same set of covariates as in the earlier analyses. Because the majority of participants completed the blood draw within 3 days from the end of the EMA period, the majority of EMA occasions occurred within 16 days of the blood draw. The forest plot in Figure 1 depicts associations based on number of days between NA assessment and the blood draw; because there was a drop off in number of occasions where the lag in days was greater than 16, these analyses focused on days that occurred within 1–16 days of the blood draw. Standardized beta estimates were typically higher when NA and biomarker assessment were closer together; for example, marginally significant associations were evident when the lag in days was between 1–4 days. A similar exploratory analysis was conducted using momentary PA; no similar trends by lag in days with momentary PA were observed.
Figure 1. Associations between NA and 7-cytokine composite based on number of days between EMA and blood draw.
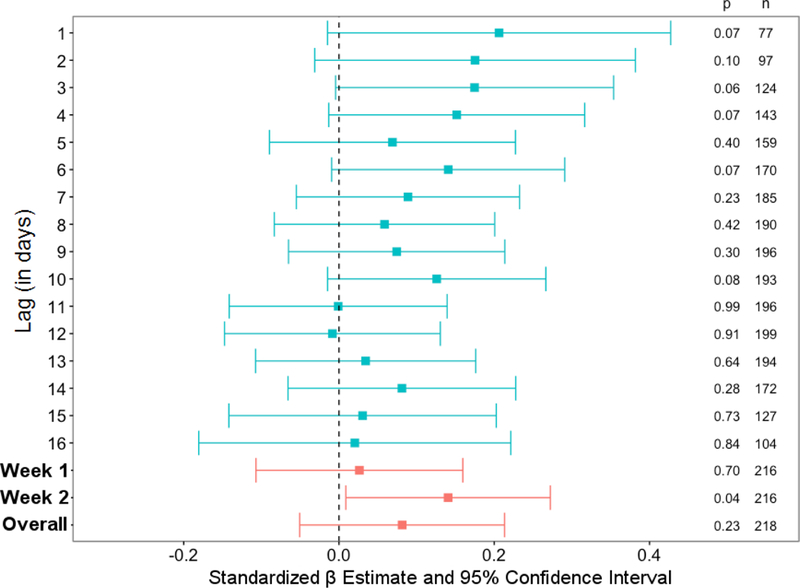
Each row represents a regression analysis with NA (aggregated across a single day) predicting the 7-cytokine composite, controlling for gender, age, BMI, chronic health conditions, statin use, and education. Confidence intervals that cross zero were non-significant. Hence, there were no significant associations observed when breaking down this association by the number of days between EMA and blood draw, although a trend was observed for stronger connections when there was a shorter lag between EMA and the blood draw.
3.5. Exploratory moderation analyses
We examined potential moderation by age, gender, race, ethnicity, and education. There was no significant moderation of any tested association between affect and inflammation by age. There was one significant moderation effect by gender. Gender moderated the association between momentary Week 2 PA and the cytokine composite (b = −0.20, p < .05); specifically, simple slopes analyses were not significant for women but, among men, lower Week 2 PA was associated with higher levels of the cytokine composite (b = 0.19, p < .05). There was also one moderation effect by Black race on the association between momentary Week 1 NA and CRP (b = −0.10, p < .05). However, simple slopes analyses were not significant for Black or non-Black participants: There was a marginally significant positive association between greater Week 1 NA and CRP among non-Black participants, b = 0.07, p = .08). There was no significant moderation by ethnicity. Finally, there was one significant moderation effect by education on the association between recalled NA and the cytokine composite (b = −0.17, p < .05): simple slopes were not significant for college graduates but, among those with less education, higher recalled NA was associated with higher levels of the cytokine composite (b = 0.16, p < .05).
4. Discussion
Our first goal was to examine associations between both recalled and momentary measures of NA and PA with measures of peripheral inflammation, with the expectation that both NA measures would be associated with higher inflammation and both PA measures would be associated with lower inflammation. Contrary to expectations, in the full sample neither recalled NA or PA nor momentary NA or PA (when aggregated across the full 14 day EMA period) was significantly associated with the 7-cytokine inflammatory composite or CRP after controlling for age, gender, BMI, education, and health-related covariates (number of chronic conditions, statin use). These null findings are consistent with a number of studies that have reported a lack of significant association between recalled affect and measures of peripheral inflammation (e.g., Andreasson et al., 2013; Friedman et al., 2007), although this literature has been mixed as reviewed earlier. Only one study to our knowledge has linked momentary PA with peripheral inflammation (IL-6 and CRP) and it did not report on NA (Steptoe et al., 2008); that study used a methodology that linked PA across one day with inflammatory measures from the same day, and significant findings were observed only among women.
Having momentary affect measures across two full weeks (collected 5x/day) enabled us to examine, as a second goal, whether the association between momentary affect and peripheral inflammation might differ based on timing. We contrasted associations when using momentary affect measures obtained closer in time to the blood draw (week 2 of the EMA) with those obtained farther from the blood draw (week 1 of the EMA), with the expectation that the former would be more robustly associated with inflammatory biomarkers due to the temporal proximity of the measures. There was support for this for NA. Whereas aggregated momentary NA from week 1 was not associated with any biomarker, aggregated momentary NA from week 2 was significantly associated with higher levels of the 7-cytokine composite measure of inflammation, controlling for age, gender, BMI, education, health conditions, and statin use. Momentary PA aggregated from either week 1 or week 2 was not associated with levels of any biomarker. To our knowledge these are the first analyses to show significant associations between aggregated momentary NA from daily life and subsequently assessed peripheral inflammation. Temporal dynamics appear to be related to these findings, as discussed further below.
4.1. Momentary versus recalled affect, and temporal dynamics
In the present research, we investigated associations between inflammation and both recalled and aggregated momentary measures of affect. We did not explicitly compare the effect sizes of associations obtained with recalled versus momentary affect because our data were not designed to enable such a test. Not only the assessment technique but the timescale varied: Our recalled affect measure assessed global affect from the past month, whereas our aggregated momentary measures encompassed a shorter time period. Thus, our recalled and momentary affect variables were of different “distances” from the blood draw from which peripheral inflammation was derived.
We were, however, able to use the density of data in our EMA assessments to examine the effects of timing for momentary affect. As described above, we showed that aggregated NA closer in time to the blood draw (Week 2) was associated with peripheral cytokines, in contrast to aggregated NA from the week before. Further, exploratory analyses examining the association between NA and the composite cytokine measure in greater detail revealed trends of association consistent with the possibility of there being stronger associations when NA assessments are taken closer in time to inflammatory assessment. Together, these findings suggest that temporal proximity was an important factor in our results. These findings also highlight the utility of EMA-derived measures, which may be particularly valuable for predicting outcomes that fluctuate over short periods of time. Levels of peripheral inflammatory markers are known to fluctuate in response to acute emotional state, perceived stress, and physical and environmental factors (Carroll et al., 2011; Marsland, Walsh, Lockwood, & John-Henderson, 2017; Steptoe et al., 2007); inflammation may thus more closely track with affect when assessments of affect are relatively recent. As discussed further below, however, our analyses examining associations between NA and the cytokine composite based on number of days between EMA and blood draw were exploratory and require replication.
Measures of recalled affect are not necessarily inferior to those of momentary affect but they are different. Assessed in daily life, momentary affect minimizes recall bias and enables questions to be asked about the relative proximity of assessment. Moreover, momentary measures may tap into a different construct than retrospective measures, perhaps one which better reflects experienced affect. This may help explain why several prior studies have found that momentary affect has predicted some physiological measures, such as measures of endocrine and cardiovascular function, more strongly (for review, see Conner & Barrett, 2012). However, although not linked to inflammation in the present research, measures of recalled PA and NA could be influential and important in other ways. For example, recalled affect measures may capture important broad self-perceptions (Kahneman & Riis, 2005; Robinson & Clore, 2002) that relate to health in a unique manner.
4.2. Null effects with CRP
No measures of affect in the present research were associated with CRP. As an end product of the inflammatory cascade, produced by the liver in response to IL-6 (Mortensen, 2001), CRP changes less rapidly than individual cytokines and may therefore not vary quickly enough within an individual to link reliably with measures of affect; CRP may better relate to more broad and stable affect-related constructs, such as hostility and depression (Graham et al., 2006). The wide range of CRP values in the present sample could be viewed as either a strength or limitation; although having greater variability in a key outcome measure is a strength, high CRP levels among our participants (many of whom had some degree of chronic illness) raises issues of generalizability (discussed at length, below). On an exploratory basis, we continued to find null effects with CRP after removing from analyses the 67 participants with CRP greater than 3 mg/L or the 39 participants with CRP greater than 10 mg/L. Moreover, we used linear regression to explore potential interaction by CRP (using a split based on >3 mg/L) on primary findings. There was a significant interaction with CRP on the association between Week 2 NA and the cytokine composite (b = 0.18, p <.05); importantly, the direction of association was the same for both those with “low” and “high” CRP, and simple slopes analyses suggested that the association between aggregated momentary NA and the cytokine composite was stronger among individuals with CRP > 3 mg/L.
4.3. Null effects with positive affect
We did not find any significant associations between PA and inflammation in the full sample. This was somewhat surprising, as PA has been related to immune function (Cohen et al., 2006) and health-related outcomes, such as pain, chronic disease incidence and progression, and mortality (Boehm & Kubzansky, 2012; Graham-Engeland, Zawadzki, Slavish, & Smyth, 2016; Pressman & Cohen, 2005; Steptoe & Wardle, 2011). However, the literature linking recalled PA and inflammation has been inconsistent and the literature linking momentary PA and inflammation scant, as reviewed above. There may be important individual differences that are related to the connection between PA and inflammation; in keeping with this, our exploratory analyses revealed a significant connection between higher momentary Week 2 PA and lower levels of the cytokine composite among males only. This is intriguing but divergent from the one other existing study to examine momentary PA and inflammation, which found a connection (in the same direction) for women only (Steptoe et al., 2008). Gender interactions such as these likely relate to complex socialized phenomena (which can vary by sample), in addition to physiological differences between men and women (Darnall & Suarez, 2009; Justeret al., 2016). Additional research structured around understanding the role of gender and other individual differences that may relate to the connection between PA and inflammation is warranted.
It also may be that constructs related to PA other than average reported PA are more important for stress physiology and inflammation. For example, we were not able to examine associations with low arousal momentary PA (such as states of being calm or pleased) versus high arousal PA (e.g., excited) using our momentary measures. This is important because emerging evidence suggests that low arousal PA may be uniquely important for health (Jones, Graham-Engeland, Smyth, & Lehman, 2018; Pressman, Jenkins, Kraft-Feil, Rasmussen, & Scheier, 2017; Schwerdtfeger, Friedrich-Mai, & Gerteis, 2015). Further, variation in PA related to daily stressors has been associated with peripheral inflammation (Sin et al., 2015). Research examining emotional variability or reactivity, or research utilizing a stress-buffering lens (e.g., Blevins et al., 2017; Jenkins, Hunter, Cross, Acevedo, & Pressman, 2018), may be needed to better understand the importance of PA on inflammation.
4.4. Interaction effects by sociodemographic factors
In addition to the gender interaction effect described above, our exploratory moderation analyses revealed two other interaction effects by the key sociodemographic factors we examined. First, in contrast to what was observed in the full sample or among college graduates (~45% of the sample), we found that among those with less than a college education there was a significant association between higher recalled NA and higher levels of the cytokine composite. This is intriguing, as it suggests that education specifically, or socioeconomic status more broadly, may help explain inconsistencies in the literature on recalled affect and inflammation. We also observed an interaction between Black race and the association between Week 1 NA and CRP, but this was harder to interpret given that there were not significant associations between either group when probed with simple slopes analyses. All of the interaction findings in the present research were exploratory and would require replication and greater probing (ideally with larger samples) prior to making any conclusions. Taken together, however, particularly in light of racial disparities in inflammation that appear to exist even beyond effects of socioeconomic status (Herd, Karraker, & Friedman, 2012; Ransome et al., 2018), these exploratory moderation analyses support the importance of having diverse samples and of examining sociodemographic differences that may relate to the connection between psychological phenomena and health.
4.5. Limitations
It may be important to consider the diverse physical and mental health of the present sample when considering generalizability. Of note, 22% of participants were flagged for at least moderate depressive symptoms (based on Patient-Reported Outcomes Measurement Information System [PROMIS] depression scores). We did not control for depressed mood or anti-depressant medication use (which is confounded with depression) because of its overlap with negative affect. Participants were not recruited on the basis of a particular health condition but neither were they excluded on the basis of most health conditions; indeed, 90% had at least one chronic health condition. Importantly, we controlled for health conditions (and statin medication use) and used winsorizing to minimize the impact of outliers on results. However, while helpful, these methods do not eliminate all potential concerns about having unhealthy participants in analyses that relate psychological and health factors. For example, there remains the possibility that a third variable (e.g., illness) might influence both affect and inflammation. At the same time, however, it is also possible that there is a causal connection from affect to inflammation that is strongest among individuals who are sick or when there is more variability in affect or inflammation. For this and several other important reasons, we elected to retain our entire potential sample. First, one of the most unique aspects of the present sample is that it was derived from systematic probability sampling of a particular geographical area (Scott et al., 2015), which resulted in sociodemographic characteristics representative of the area. Although no sample can generalize to the entire population, this sampling technique mitigates concerns regarding generalizability in ways that most studies do not. Moreover, the present sample was comprised of a majority of African-American participants and a sizable minority of Hispanic participants. Recent studies have revealed significantly higher CRP levels among African-Americans compared to White participants (Gruenewald, Cohen, Matthews, Tracy, & Seeman, 2009; Herd et al., 2012; Ranjitet al., 2007; Ransome et al., 2018). Thus, the present sample may be more representative of at least certain populations than many other studies, which frequently exclude individuals on the basis of health conditions and which are often predominately White.
Our analyses testing interaction effects and some of those focused on the temporal dynamics related to momentary affect were exploratory and will require replication. Further, we were not able to distinguish the specific and relative contributions of assessment method (recall versus EMA) due to differences in the duration of recall (e.g., affect over a month versus a week) and in the proximity to blood sampling (i.e., we did not have recalled measures proximal to the blood draw). Due to limitations in our assessment of affect, we were also unable to test the unique contribution of low versus high arousal NA and PA. These are exciting questions that remain for future research. In addition, the data analyzed in the present research were cross-sectional; hence, these findings do not demonstrate directionality between affect and inflammation. Existing experimental research suggests that there are bi-directional pathways between emotion and inflammation (e.g., Brydon, Walker, Wawrzyniak, Chart, & Steptoe, 2009; Carroll et al., 2011; Moons, Eisenberger, & Taylor, 2010; Wright, Strike, Brydon, & Steptoe, 2005). Future research is needed to better characterize the many potential mechanisms for associations between affect and inflammation, which include stress activation and behavioral pathways. Research that adds additional inflammatory time points (e.g., a blood draw both before and after an EMA period or multiple inflammatory assessments via dried blood spots or saliva) would be valuable to better characterize both the directionality and temporality of these associations.
4.6. Conclusions
This research adds to the literature suggesting that affect itself (as opposed to broader constructs involving affect [e.g., hostility]) can relate to markers of inflammation. Although recalled affect was not significantly linked with inflammation (except among those with lower education), momentary negative affect in daily life (from the week prior to the blood draw only) was associated with inflammation based on a panel of cytokines. Further, momentary positive affect (again, only from the week prior to the blood draw) was associated with the same set of inflammatory cytokines but among males only. One broad possibility suggested by this research is that momentary affect measures more proximal to blood sampling may be stronger predictors of inflammatory markers. Together, the present findings suggest that a) it may be valuable to include momentary measures of affect in research linking affect with inflammation, and b) affect measures obtained closer in time to blood draw(s) may associate more robustly with peripheral inflammation. Further investigation of these nuanced questions, involving assessment techniques and temporal dynamics, is needed to more fully understand the important associations between emotion and inflammation.
Supplementary Material
Highlights.
Aggregated momentary negative affect and inflammation were significantly associated
Recalled affect measures were not associated with inflammation in the same sample
Momentary affect measures proximal to blood sampling may be stronger predictors
The sample comprised racially and socioeconomically diverse mid-life adults
Sociodemographic factors were explored as moderators of effects
Acknowledgments
Notes and Acknowledgements: Sin is now in the Department of Psychology at the University of British Columbia. Work on this project was supported by National Institute of Health (NIH) grants R01 AG039409 (Sliwinski), R01 AG042595 (Graham-Engeland and Engeland), P01-AG0394 (Lipton), and F32AG048698 (Sin). Knight and Jones were partially supported by NIA T32AG049676 to The Pennsylvania State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasson AN, Szulkin R, Unden AL, von Essen J, Nilsson LG, & Lekander M (2013). Inflammation and positive affect are associated with subjective health in women of the general population. J Health Psychol, 18(3), 311–320. 10.1177/1359105311435428 [DOI] [PubMed] [Google Scholar]
- Bajaj A, John-Henderson NA, Cundiff JM, Marsland AL, Manuck SB, & Kamarck TW (2016). Daily social interactions, close relationships, and systemic inflammation in two samples: Healthy middle-aged and older adults. Brain Behav Immun, 58, 152–164. 10.1016/j.bbi.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins CL, Sagui SJ, & Bennett JM (2017). Inflammation and positive affect: Examining the stress-buffering hypothesis with data from the National Longitudinal Study of Adolescent to Adult Health. Brain Behav Immun, 61, 21–26. 10.1016/j.bbi.2016.07.149 [DOI] [PubMed] [Google Scholar]
- Boehm JK, & Kubzansky LD (2012). The heart’s content: the association between positive psychological well-being and cardiovascular health. Psychol Bull, 138(4), 655–691. 10.1037/a0027448 [DOI] [PubMed] [Google Scholar]
- Brydon L, Walker C, Wawrzyniak AJ, Chart H, & Steptoe A (2009). Dispositional optimism and stress-induced changes in immunity and negative mood. Brain Behav Immun, 23(6), 810–816. 10.1016/j.bbi.2009.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Low CA, Prather AA, Cohen S, Fury JM, Ross DC, & Marsland AL (2011). Negative affective responses to a speech task predict changes in interleukin (IL)-6. Brain Behav Immun, 25(2), 232–238. 10.1016/j.bbi.2010.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Alper CM, Doyle WJ, Treanor JJ, & Turner RB (2006). Positive emotional style predicts resistance to illness after experimental exposure to rhinovirus or influenza a virus. Psychosom Med, 68(6), 809–815. 10.1097/01.psy.0000245867.92364.3c [DOI] [PubMed] [Google Scholar]
- Conen D, & Ridker PM (2007). Clinical significance of high-sensitivity C-reactive protein in cardiovascular disease. Biomark Med, 1(2), 229–241. 10.2217/17520363.1.2.229 [DOI] [PubMed] [Google Scholar]
- Conner TS, & Barrett LF (2012). Trends in ambulatory self-report: The role of momentary experience in psychosomatic medicine. Psychosom Med, 74(4), 327–337. 10.1097/PSY.0b013e3182546f18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranford JA, Shrout PE, Iida M, Rafaeli E, Yip T, & Bolger N (2006). A Procedure for evaluating sensitivity to within-person change: Can mood measures in diary studies detect change reliably? Personality & Social Psychology Bulletin, 32(7), 917–929. 10.1177/0146167206287721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly M (2012). Are momentary measures of positive affect better predictors of mortality than recalled feelings? Proc Natl Acad Sci, 109, E1049–E1049. 10.1073/pnas.1201630109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall BD, & Suarez EC (2009). Sex and gender in psychoneuroimmunology research: Past, present and future. Brain Behav Immun, 23(5), 595–604. 10.1016/j.bbi.2009.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn LD, Gayles JG, Engeland CG, Houts R, Cizza G, & Denson LA (2016). Cytokine Patterns in Healthy Adolescent Girls: Heterogeneity captured by variable and person-centered statistical strategies. Psychosom Med, 78(6), 646–656. 10.1097/psy.0000000000000321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Harris T, Guralnik J, Tracy R, Corti M, Cohen H, . . . Havlik R (1999). Serum IL-6 level and the development of disability in older persons. J Am Geriatric Soc, 47, 639–646. [DOI] [PubMed] [Google Scholar]
- Friedman EM, Hayney M, Love GD, Singer BH, & Ryff CD (2007). Plasma interleukin-6 and soluble IL-6 receptors are associated with psychological well-being in aging women. Health Psychol, 26(3), 305–313. 10.1037/0278-6133.26.3.305 [DOI] [PubMed] [Google Scholar]
- Graham-Engeland JE, Zawadzki MJ, Slavish DC, & Smyth JM (2016). Depressive symptoms and momentary mood predict momentary pain among rheumatoid arthritis patients. Ann Behav Med, 50(1), 12–23. 10.1007/s12160-015-9723-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JE, Christian LM, & Kiecolt-Glaser JK (2006). Stress, age, and immune function: Toward a lifespan approach. J Behav Med, 29(4), 389–400. 10.1007/s10865-006-9057-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JE, Robles TF, Kiecolt-Glaser JK, Malarkey WB, Bissell MG, & Glaser R (2006). Hostility and pain are related to inflammation in older adults. Brain Behav Immun, 20(4), 389–400. 10.1016/j.bbi.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Cohen S, Matthews KA, Tracy R, & Seeman TE (2009). Association of socioeconomic status with inflammation markers in black and white men and women in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Soc Sci Med, 69(3), 451–459. 10.1016/j.socscimed.2009.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett RA, Lazzarino AI, Carvalho LA, Hamer M, & Steptoe A (2015). Hostility and physiological responses to acute stress in people with type 2 diabetes. Psychosom Med, 77(4), 458–466. 10.1097/psy.0000000000000172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd P, Karraker A, & Friedman E (2012). The social patterns of a biological risk factor for disease: race, gender, socioeconomic position, and C-reactive protein. J Gerontol B Psychol Sci Soc Sci, 67(4), 503–513. 10.1093/geronb/gbs048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M (2001). Depression and immunity. In Ader R, Felten DL, & Cohen N (Eds.), Psychoneuroimmunology, Third Edition (Vol. 2, pp. 383–398). San Diego: Academic Press. [Google Scholar]
- Jenkins BN, Hunter JF, Cross MP, Acevedo AM, & Pressman SD (2018). When is affect variability bad for health? The association between affect variability and immune response to the influenza vaccination. J Psychosom Res, 104, 41–47. 10.1016/j.jpsychores.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DR, Graham-Engeland JE, Smyth JM, & Lehman BJ (2018). Clarifying the associations between mindfulness meditation and emotion: Daily high-and low-arousal emotions and emotional variability. Appl Psychol Health Well Being 10.1111/aphw.12135 [DOI] [PubMed] [Google Scholar]
- Juster RP, Pruessner JC, Desrochers AB, Bourdon O, Durand N, Wan N, . . . Lupien SJ (2016). Sex and gender roles in relation to mental health and allostatic load. Psychosom Med, 78(7), 788–804. 10.1097/psy.0000000000000351 [DOI] [PubMed] [Google Scholar]
- Kahneman D, & Riis J (2005). Living, and thinking about it: Two perspectives on life. In Huppert FA, Baylis N, & Keverne B (Eds.), The science of well-being (pp. 285–304): Oxford University Press. [Google Scholar]
- Kamarck TW, Schwartz JE, Shiffman S, Muldoon MF, Sutton-Tyrrell K, & Janicki DL (2005). Psychosocial stress and cardiovascular risk: What is the role of daily experience? J Pers, 73(6), 1749–1774. 10.1111/j.0022-3506.2005.00365.x [DOI] [PubMed] [Google Scholar]
- Marsland AL, Prather AA, Petersen KL, Cohen S, & Manuck SB (2008). Antagonistic characteristics are positively associated with inflammatory markers independently of trait negative emotionality. Brain Behav Immun, 22(5), 753–761. 10.1016/j.bbi.2007.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Walsh C, Lockwood K, & John-Henderson NA (2017). The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav Immun, 64, 208–219. 10.1016/j.bbi.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, & Raison CL (2016). The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat Rev Immunol, 16(1), 22–34. 10.1038/nri.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Freedland KE, Carney RM, Stetler CA, & Banks WA (2003). Cynical hostility, depressive symptoms, and the expression of inflammatory risk markers for coronary heart disease. J Behav Med, 26, 501–515. 10.1023/A:1026273817984 [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Boylan JM, Coe CL, Curhan KB, Levine CS, Markus HR, . . . Ryff CD (2013). Negative emotions predict elevated interleukin-6 in the United States but not in Japan. Brain Behav Immun, 34, 79–85. 10.1016/j.bbi.2013.07.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montpetit MA, Bergeman CS, Deboeck PR, Tiberio SS, & Boker SM (2010). Resilience-as-process: Negative affect, stress, and coupled dynamical systems. Psychol Aging, 25(3), 631–640. 10.1037/a0019268 [DOI] [PubMed] [Google Scholar]
- Moons WG, Eisenberger NI, & Taylor SE (2010). Anger and fear responses to stress have different biological profiles. Brain Behav Immun, 24(2), 215–219. 10.1016/j.bbi.2009.08.009 [DOI] [PubMed] [Google Scholar]
- Mortensen RF (2001). C-reactive protein, inflammation, and innate immunity. Immunol Res, 24(2), 163–176. 10.1385/ir:24:2:163 [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, . . . Irwin MR (2009). To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun, 23(7), 887–897. 10.1016/j.bbi.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Out D, Hall RJ, Granger DA, Page GG, & Woods SJ (2012). Assessing salivary C-reactive protein: Longitudinal associations with systemic inflammation and cardiovascular disease risk in women exposed to intimate partner violence. Brain Behav Immun, 26(4), 543–551. 10.1016/j.bbi.2012.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan A, Manson J, Rifai N, Buring J, & Ridker P (2001). C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA, 286, 327–334. [DOI] [PubMed] [Google Scholar]
- Pressman SD, & Cohen S (2005). Does positive affect influence health? Psychol Bull, 131(6), 925–971. 10.1037/0033-2909.131.6.925 [DOI] [PubMed] [Google Scholar]
- Pressman SD, Jenkins BN, Kraft-Feil TL, Rasmussen H, & Scheier MF (2017). The whole is not the sum of its parts: Specific types of positive affect influence sleep differentially. Emotion, 17(5), 778–793. 10.1037/emo0000256 [DOI] [PubMed] [Google Scholar]
- Pripp AH, & Stanisic M (2014). The correlation between pro-and anti-inflammatory cytokines in chronic subdural hematoma patients assessed with factor analysis. PLoS ONE, 9(2), e90149 10.1371/journal.pone.0090149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjit N, Diez-Roux AV, Shea S, Cushman M, Ni H, & Seeman T (2007). Socioeconomic position, race/ethnicity, and inflammation in the multi-ethnic study of atherosclerosis. Circulation, 116(21), 2383–2390. 10.1161/circulationaha.107.706226 [DOI] [PubMed] [Google Scholar]
- Ransome Y, Slopen N, Karlsson O, & Williams DR (2018). Elevated inflammation in association with alcohol abuse among Blacks but not Whites: results from the MIDUS biomarker study. J Behav Med, 41(3), 374–384. 10.1007/s10865-017-9905-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, & Clore GL (2002). Belief and feeling: evidence for an accessibility model of emotional self-report. Psychol Bull, 128(6), 934–960. [DOI] [PubMed] [Google Scholar]
- Schwarz N (2007). Retrospective and concurrent self-reports: The rationale for real-time data capture. In Stone A, Shiffman S, Atienza A, & Nebeling L (Eds.), The science of real-time data capture: Self-reports in health research (pp. 11–26). New York: Oxford University Press. [Google Scholar]
- Schwerdtfeger AR, Friedrich-Mai P, & Gerteis AK (2015). Daily positive affect and nocturnal cardiac activation. Int J Behav Med, 22(1), 132–138. 10.1007/s12529-014-9396-4 [DOI] [PubMed] [Google Scholar]
- Scott SB, Graham-Engeland JE, Engeland CG, Smyth JM, Almeida DM, Katz MJ, . . . Sliwinski MJ (2015). The Effects of Stress on Cognitive Aging, Physiology and Emotion (ESCAPE) project. BMC Psychiatry, 15(1), 1–14. 10.1186/s12888-015-0497-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, & Hufford MR (2008). Ecological momentary assessment. Annu Rev Clin Psychol, 4, 1–32. [DOI] [PubMed] [Google Scholar]
- Sin NL, Graham-Engeland JE, Ong AD, & Almeida DM (2015). Affective reactivity to daily stressors is associated with elevated inflammation. Health Psychol, 34, 1154–1165. 10.1037/hea0000240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JM, & Heron KE (2012). Health Psychology. In Conner MRMTS (Ed.), Handbook of Research Methods for Studying Daily Life (pp. 569–584). New York: The Guilford Press. [Google Scholar]
- Steptoe A, Gibson EL, Hamer M, & Wardle J (2007). Neuroendocrine and cardiovascular correlates of positive affect measured by ecological momentary assessment and by questionnaire. Psychoneuroendocrinology, 32(1), 56–64. 10.1016/j.psyneuen.2006.10.001 [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, & Chida Y (2007). The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behav Immun, 21(7), 901–912. 10.1016/j.bbi.2007.03.011 [DOI] [PubMed] [Google Scholar]
- Steptoe A, O’Donnell K, Badrick E, Kumari M, & Marmot M (2008). Neuroendocrine and inflammatory factors associated with positive affect in healthy men and women: The Whitehall II study. Am J Epidemiol, 167(1), 96–102. 10.1093/aje/kwm252 [DOI] [PubMed] [Google Scholar]
- Steptoe A, & Wardle J (2011). Positive affect measured using ecological momentary assessment and survival in older men and women. Proc Natl Acad Sci U S A, 108(45), 18244–18248. 10.1073/pnas.1110892108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AA, Neale JM, Cox DS, Napoli A, Valdimarsdottir H, & Kennedy-Moore E (1994). Daily events are associated with a secretory immune response to an oral antigen in men. Health Psychol, 13(5), 440–446. [DOI] [PubMed] [Google Scholar]
- Stoner L, Lucero AA, Palmer BR, Jones LM, Young JM, & Faulkner J (2013). Inflammatory biomarkers for predicting cardiovascular disease. Clin Biochem, 46(15), 1353–1371. 10.1016/j.clinbiochem.2013.05.070 [DOI] [PubMed] [Google Scholar]
- Sturgeon JA, Arewasikporn A, Okun MA, Davis MC, Ong AD, & Zautra AJ (2016). The psychosocial context of financial stress: Implications for inflammation and psychological health. Psychosom Med, 78(2), 134–143. 10.1097/psy.0000000000000276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez EC, Lewis JG, Krishnan RR, & Young KH (2004). Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology, 29(9), 1119–1128. 10.1016/j.psyneuen.2004.01.002 [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, & Fidell LS (2001). Using Multivariate Statistics 4th Edition Boston: Allyn and Bacon. [Google Scholar]
- Wright CE, Strike PC, Brydon L, & Steptoe A (2005). Acute inflammation and negative mood: Mediation by cytokine activation. Brain Behav Immun, 19(4), 345–350. 10.1016/j.bbi.2004.10.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


