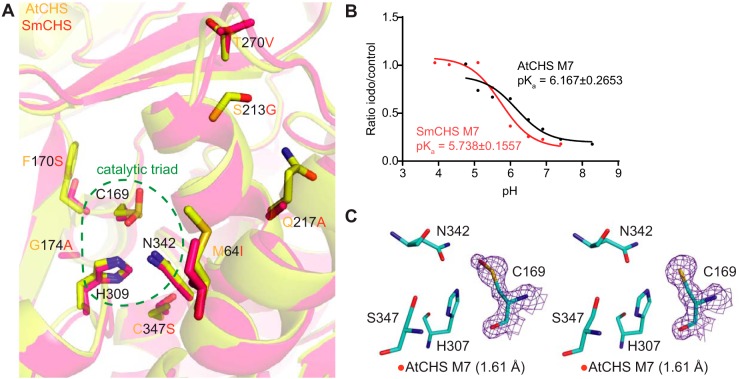
Figure 4.
Identification and characterization of additional key residues that affect CHS cysteine reactivity. A, overlaid crystal structures of AtCHS and SmCHS showing the seven conserved residue differences between euphyllophyte and basal-plant CHSs. B, pKa measurement of AtCHS M7 and SmCHS M7 mutants. The pKa of each M7 mutant is about 0.5 pH units higher or lower, respectively, than the corresponding WT CHS. C, active sites of the two monomers of the AtCHS M7 septuple mutant structure. The 2Fo − Fc electron density map contoured at 1.5σ is shown around the catalytic cysteine. The crystal structure shows oxidation to sulfenic acid in the catalytic cysteine of one chain (left) and a reduced cysteine in the other (right).