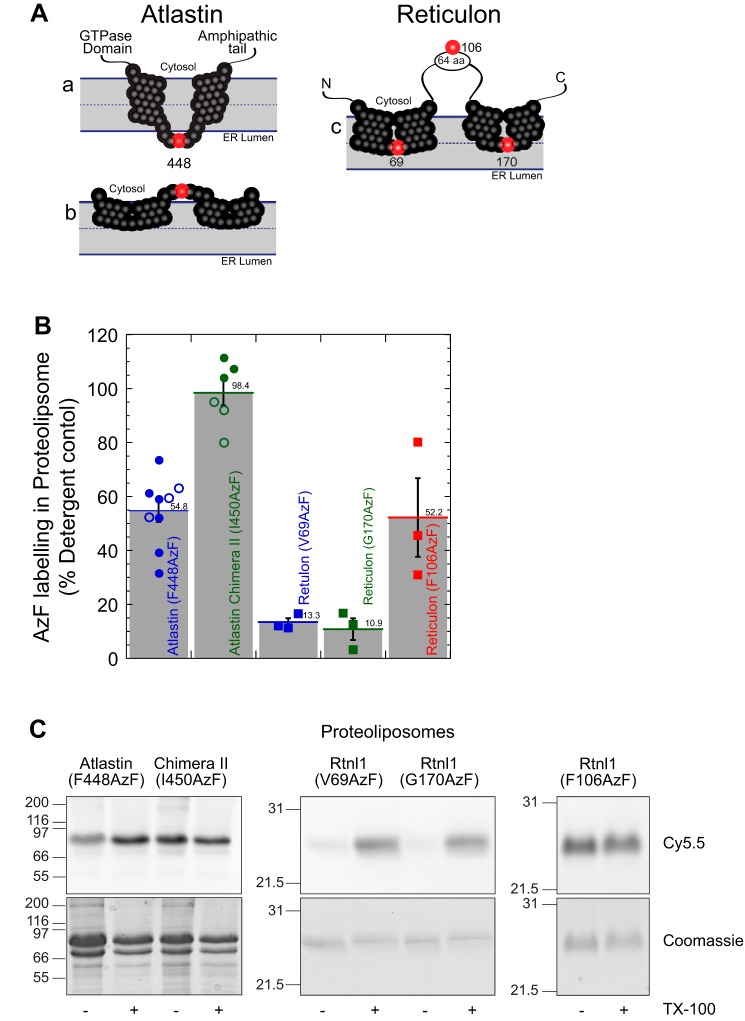
Figure 4.
The atlastin membrane domain is a dual-hairpin loop that does not span the lipid bilayer. A, schematic representation of the putative conformations of atlastin and reticulon membrane domain. A (a), two transmembrane segments that traverse the lipid bilayer. A (b), two hairpin loops that are inserted into the outer bilayer. Phenylalanine 448 is represented as a red sphere, and the other residues in the membrane domain are shown as black spheres. A (c), schematic representation of reticulon homology domain with valine 69, phenylalanine 106, and glycine 170 represented with red spheres. B, proteoliposomes labeling of atlastin and reticulon azidophenylalanine mutants with DBCO-Cy5.5 by copper-free click chemistry. Following SDS-PAGE, Cy5.5 fluorescence in each band was analyzed and normalized to its corresponding Coomassie-stained band. Samples were then also normalized to detergent-solubilized proteoliposomes. Filled symbols, proteoliposomes following isolation by flotation in a density gradient; open symbols, proteoliposomes quenched with excess NaN3. C, representative SDS-PAGE samples of each mutant in proteoliposomes (− TX-100) or detergent micelles (+ TX-100) visualizing Cy5.5 fluorescence (top) and total protein by Coomassie staining (bottom). TX-100, Triton X-100. Error bars indicate S.E.