Figure 3.
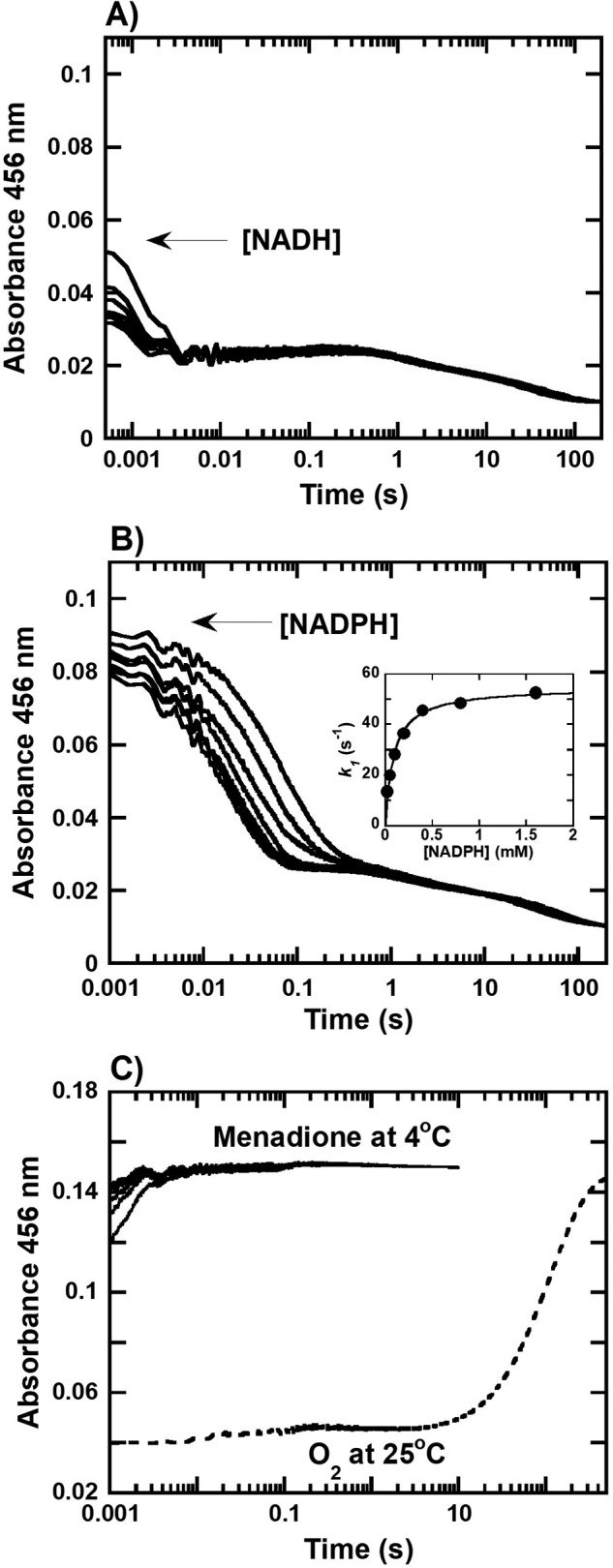
Transient kinetics of the reductive and oxidative half-reaction of HadB. A and B, kinetic traces of the reactions of anaerobic HadBFMN (12 μm) mixing with (A) NADH or (B) NADPH (0.05–3.2 mm) in 50 mm NaPi, pH 7.0, at 4 °C. Inset in B shows a plot between the observed rate constant (kobs) of the first phase of the reaction versus NADPH concentrations which yields a hyperbolic relationship. C, kinetic traces of the reactions of anaerobic HadBFMNH− (12.5 μm) mixing with various concentrations of menadione (50–200 μm) in 50 mm NaPi, pH 7.0, at 4 °C (solid lines) and reactions of HadBFMNH− (12.5 μm) mixed with O2 (0.13 mm) in 50 mm NaPi, pH 7.0, at 25 °C (dashed lines) were obtained by monitoring the absorbance of the reactions at 456 nm.
