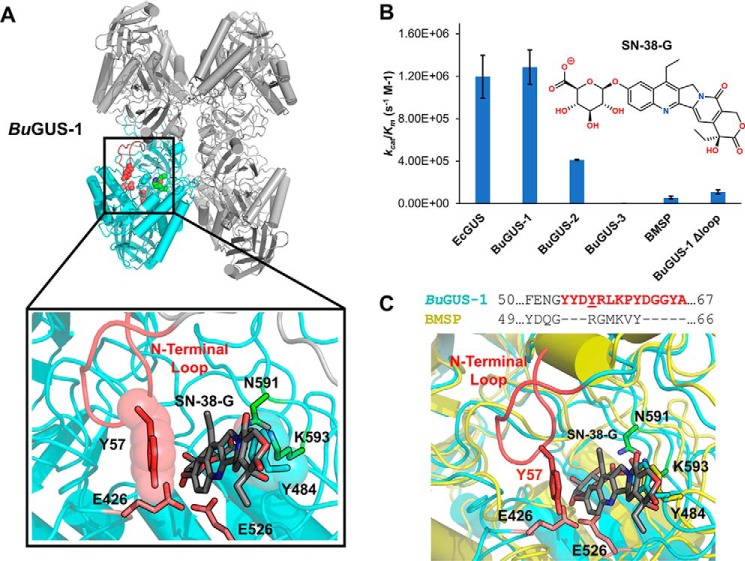
Figure 8.
Kinetic and structural analysis of SN-38-G hydrolysis reveals the importance of the N-terminal loop in BuGUS-1. A, BuGUS-1 tetramer with adjacent N-terminal loop highlighted in red, catalytic glutamates in deep salmon, and the NXK motif in green. Zoom-in of active site with SN-38-G manually docked in the active site of BuGUS-1 based on the PTG-bound structure. B, catalytic efficiencies kcat/Km for EcGUS, BuGUS-1, BuGUS-2, BuGUS-3, BMSP, and BuGUS-1 Δloop with the substrate SN-38-G. C, sequence alignment of BuGUS-1 and BMSP GUS N-terminal loop regions and overlay of BuGUS-1 and BMSP active sites with SN-38-G manually docked. Error bars represent S.D. of n = 3 biological replicates.