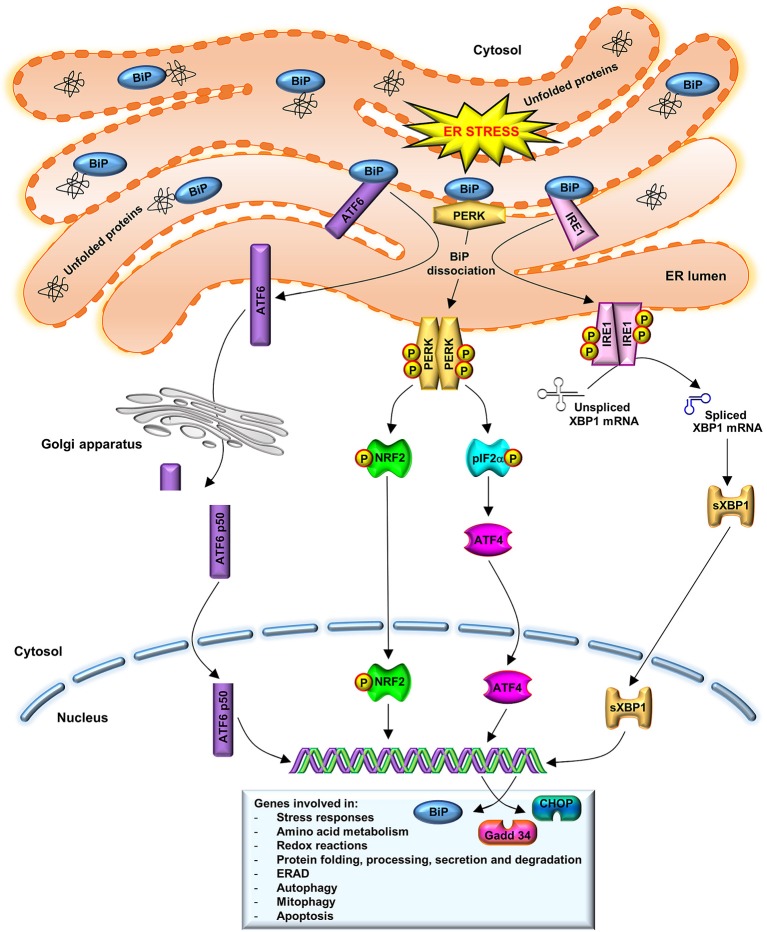
Figure 1.
UPR activation. BiP dissociation from PERK, IRE1, and ATF6 initiates UPR through both PERK and IRE1 oligomerization and activation via trans-autophosphorylation and ATF6 translocation to the Golgi complex. Activated PERK phosphorylates eIF2a and stimulates ATF4 activity, which induces ERS target genes that are involved in amino acid metabolism, redox reactions, and protein secretion, thus to promote cell survival. A prolonged ATF4 activation leads to induction of CHOP. IRE1 dimerization catalyzes the splicing of XBP1 mRNA to synthetize a 54 kDa protein (sXBP1) which induces the expression of chaperones, as BiP, and components of the ERAD pathway. BiP also participates in cellular process such as autophagy, mitophagy, and apoptosis. In order to restore normal ER function, new synthesized BiP binds to PERK, IRE1, and to unfolded proteins, to refold them. ATF6 translocates to the Golgi, where it is cleaved in an active N-terminal 50 kDa domain. This active fragment is translocated to the nucleus where upregulates ER-associated chaperones and protein degradation factors, as well as CHOP and XBP1 expression.