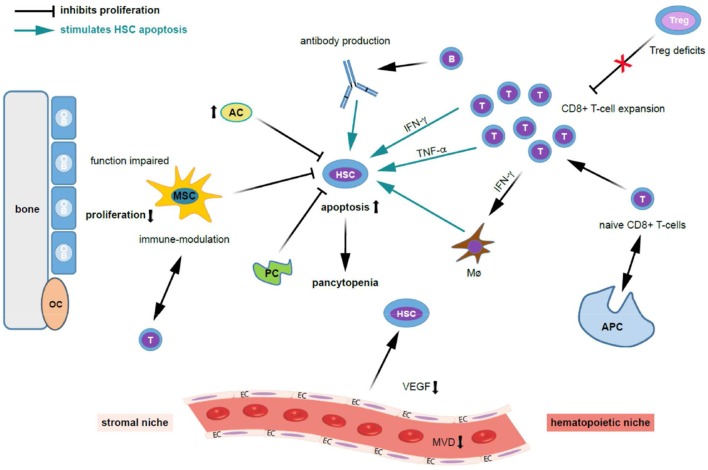
Figure 2.
Possible mechanisms contributing to bone marrow niche modulation and immune destruction of hematopoiesis in acquired aplastic anemia. Patients with acquired aplastic anemia (AA) display not only low numbers of hematopoietic stem cells (HSC) but also an altered hematopoietic niche. On the left side of the figure the effect of stromal cells (“stromal niche”) and its interaction with HSC and on the right side the effects of the immune cells on HSC (“hematopoietic niche”) are shown. Regarding the auto-immune pathophysiology in acquired AA, antigens are presented to naive CD8+ T cells by antigen presenting cells (APCs), which trigger T cells to activate and proliferate. Cytotoxic T cells (a polyclonal expansion of dysregulated CD4+ T-cells) triggering apoptosis in bone marrow (BM) cells. Further, activated T lymphocytes induce apoptosis in HSCs and oligoclonal expansion of dysregulated CD8+ T-cell populations. Besides that, there is abnormal production of cytokines including interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF- α), and transforming growth factor (TGF) which induces HSC apoptosis through Fas and the Fas ligand. These events ultimately lead to reduced cell cycling and HSC cell death by apoptosis. Quantitative and qualitative deficits of regulatory T cells (Tregs), which normally suppress auto-reactivity of other T cell populations, further stimulates T cell expansion. TNF-α-producing macrophages (Mø) in the BM were more frequent in AA patients. Further, IFN-γ-mediated HSC loss was shown to require the presence of Mø. INF-γ increases BM Mø which drives loss of megakaryocytes and HSC. The potential for IFN-γ to both directly exhaust and deplete HSCs, as well as to indirectly reduce HSC function through microenvironmental niche cells, particularly Mø, and mesenchymal stem cells (MSCs), adds complexity to the study of AA pathogenesis. Possibly, B cells, which are increased in AA patients, produce auto-antibodies against HSC. Regarding the stromal niche, impairments in osteoblastic, vascular, and perivascular HSC niches might contribute to defective hematopoiesis in patients with AA. MSC function is impaired in AA, HSCs cannot adequately proliferate, and activated T-cells are not suppressed. MSC aberrant alteration impair the maintaining of the immune homeostasis. Adipocytes (AC) are increased and pericytes are decreased (PC) and suppress hematopoiesis. Further, the microvessel density (MVD) and vascular endothelial growth factor (VEGF) expression is decreased in AA. Given the close interaction and regulatory feedback loops between resident hematopoietic and niche cells, it is not surprising that besides immune destruction, AA also associates with defects in non-hematopoietic BM microenvironment components. AC, adipocytes; APC, antigen-presenting cell; HSC, hematopoietic stem cell; EC, endothelial cells; INF-γ, interferon-gamma; MVD, microvessel density; Mø, macrophages; MSC, mesenchymal stem cells; OB, osteoblasts; OC, osteoclasts; PC, pericytes; TNF-α, tumor necrosis factor-alpha; VEGF, vascular endothelial growth factor.