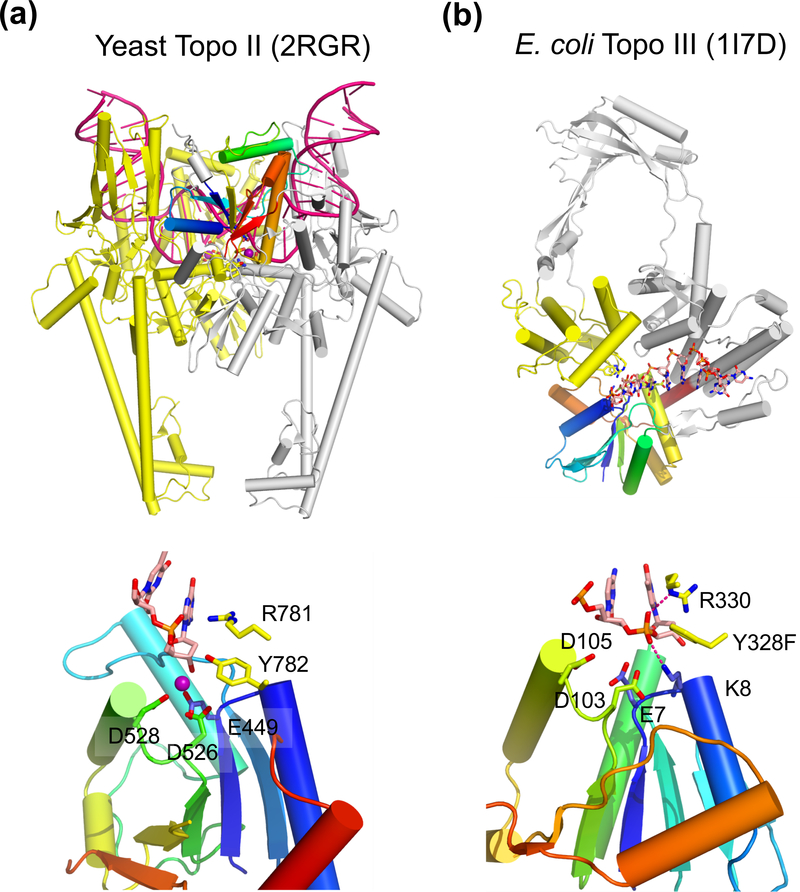
Fig. 5.
Structure of type II and type IA topoisomerase. (a) The overall structure of the dimeric yeast Topo II (type II) bound to a substrate DNA (G segment, PDB: 2RGR). One subunit is shown in silver with the TOPRIM domain in rainbow colors. The other subunit, which donates the tyrosine nucleophile to the rainbow-colored TOPRIM, is shown in yellow. The DNA substrate is severely bent and shown in dark pink. The N-terminal ATPase domain is absent in the crystal structure (not shown) and would be on top of DNA in this view. (b) E. coli Topo III (type IA, PDB: 1I7D) is monomeric. The TOPRIM is shown in rainbow colors. The tyrosine nucloephile is donated by the yellow domain. The rest of the protein is colored in silver, The ssDNA substrate is shown as multicolored sticks. The active site of Topo II and Topo III are shown below. In addition to the three carboxylates (E, DxD), the catalytically essential Arg and Tyr (nucleophile) are donated from the second subunit or domain (colored yellow) for catalysis. The Mg2+ found in Topo II is shown as a purple sphere.