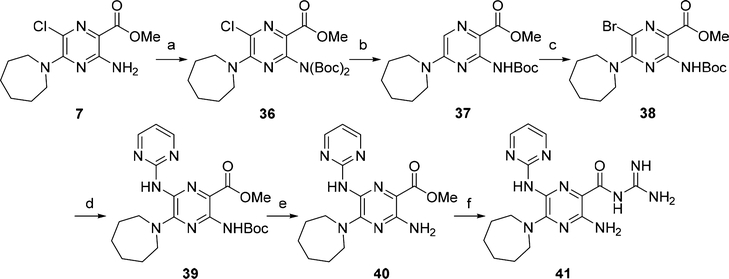
Scheme 3. Synthesis of 2-Aminopyrimidinyl Derivative 41a.
aReagents and conditions: (a) (Boc)2O, CH2Cl2, DIPEA, DMAP, rt, 16 h, 85%; (b) Pd/C, H2 (1 atm), MgO, MeOH, rt, 48 h, 92%; (c) N-bromosuccinimide, CH2Cl2, rt, 5 h, 60%; (d) 2-aminopyrimidine, Pd2(dba)3/Xantphos, 1,4-dioxane, Cs2CO3, 100 °C, 18 h, 30%; (e) TFA, CH2Cl2, rt, 16 h, 98%; (f) guanidine (2 M in MeOH), DMF, rt, 35%.