To the Editor:
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide. Cigarette smoking is an important risk factor for COPD; however, not all heavy smokers develop COPD. In fact, the majority of smokers do not develop airflow limitation consistent with COPD, whereas 25–35% appear to be susceptible, deteriorating into clinical COPD at a young age (1, 2). COPD severity and progression in patients younger than 50 years is similar to that of patients older than 65 years (3), suggesting that the process begins much earlier in life in subjects at risk for the disease. In support of this concept, Lange and colleagues showed that the combination of low baseline lung function (LF) and rapid LF decline, as defined by mean annual loss of 40 ml FEV1 or more (rapid decline [RD]), results in “impending” development of COPD compared with subjects having only one or neither of the conditions (4). We tested the hypothesis that middle-aged and heavy smokers with low LF and rapid LF decline trajectory have a high hazard ratio to develop COPD at an earlier time than smokers with other LF trajectories.
Since 2001, the Lovelace Smokers Cohort (LSC), a community-based cohort of volunteers, has been recruiting heavy current and former smokers (≥10 pack-years) with ages ranging from 40 to 75 years. These smokers are either at risk for development of COPD or have already acquired the disease. Details regarding the LSC have been published previously (5, 6). The LSC captures questionnaire, demographic, and pre- and postbronchodilator spirometry data. Visits occur at 18-month intervals, and LF change can be characterized over time. In this study, we sought to investigate the incidence and factors involved in the development of spirometric Global Initiative for Obstructive Lung Disease stage II COPD in smokers at risk (32.4 mean pack-years) aged 40 to 50 years and followed for a mean period of 4 years. COPD in subjects younger than 50 years of age with 10 pack-years or more was recently termed early COPD (7)
Study participants were selected on the basis of their LF trajectories, grouped according to the combination of baseline LF and LF decline status. From the initial cohort of 2,273 LSC volunteers, 1,553 subjects had a minimum of two visits used to determine LF change, as previously reported (8). From these subjects, we selected those aged 40–50 years at baseline (n = 685). These subjects were grouped into tertiles of baseline LF, as well as into tertiles of annual rate of FEV1 change between baseline and most recent spirometry measurements. Subjects falling into the middle tertile of either baseline LF or LF change were excluded. Therefore, the four comparison groups comprised participants with high or low baseline LF, each with no decline (ND) or RD in LF.
All groups were similar in age, sex, socioeconomic status (educational attainment), pack-years, and current smoking status. Subjects in the low LF/RD group were slightly older than those in the other groups, and were more likely to demonstrate bronchodilator reversibility, but no differences were observed with respect to exposure to pollutants, parental smoking, comorbidities, or medication use.
Participants in the low LF/RD group had the highest prevalence of baseline COPD and the lowest FEV1/FVC ratio (Table 1). The proportion of subjects with a modified Medical Research Council dyspnea score ≥2 was the highest in the low LF/RD group. St. George’s Respiratory Questionnaire total scores and symptom, activity, and impact subscale scores were highest, and 36-item Short Form Questionnaire scores lowest (indicating worse health status), in the low LF/RD group. The proportion of New Mexico Hispanics was higher in the high baseline LF groups, in keeping with other studies that have reported LF protection for this ethnic group (5). However, excluding Hispanics from these analyses did not change any of the demographic or clinical results shown in Table 1. Most important, incident COPD was significantly higher in subjects with low LF/RD, and the mean number of days to incident COPD was 0.33–0.5 times the interval for incident COPD seen in the other groups. After adjusting for age, sex, body mass index, socioeconomic status, pack-years, and current smoking status, the relative risk for incident COPD was significantly higher, with a hazard ratio of 36.6 (95% confidence interval, 4.1–320.9) in subjects with low LF/RD compared with subjects with high LF/ND. The relative risk for the other groups was not significantly increased.
Table 1.
Comparison of Selected Lung Function Trajectory Groups among Young Smokers (40–50 yr of Age) in the Lovelace Smokers’ Cohort (N = 222)
| Characteristics | ND |
RD |
P Value | ||
|---|---|---|---|---|---|
| High LF (n = 40) | Low LF (n = 70) | High LF (n = 68) | Low LF (n = 44) | ||
| Age, yr | 44.6 ± 3.2 | 45.4 ± 3.9 | 44.8 ± 3.1 | 46.7 ± 3.2 | 0.03 |
| Male | 10 (25) | 21 (30) | 18 (26.5) | 7 (15.9) | 0.26 |
| Hispanic | 15 (37.5) | 21 (30) | 16 (23.5) | 6 (13.6) | 0.01 |
| BMI, kg/m2 | 27.6 ± 6.3 | 30.6 ± 8 | 26.9 ± 5.2 | 27.2 ± 6.4 | 0.13 |
| Education, ≥HS | 23 (57.5) | 47 (67.1) | 54 (79.4) | 23 (52.3) | 0.92 |
| Current smoker | 30 (75) | 56 (80) | 45 (66.2) | 34 (77.3) | 0.56 |
| Pack-year | 30.2 ± 13.7 | 34.5 ± 15.8 | 28 ± 10.2 | 36.9 ± 16.4 | 0.34 |
| Exposure to fumes | 14 (35) | 19 (27.5) | 19 (27.9) | 14 (32.6) | 0.87 |
| Exposure to dust | 6 (15) | 6 (8.7) | 5 (7.4) | 9 (20.9) | 0.48 |
| Wood smoke exposure | 10 (25.6) | 20 (29) | 19 (28.4) | 16 (37.2) | 0.31 |
| Follow-up, yr | 4.1 ± 2.1 | 4.2 ± 2.4 | 4.3 ± 2.5 | 3.3 ± 2.3 | 0.19 |
| Visits, n | 4 ± 1.4 | 4 ± 1.5 | 4 ± 1.5 | 3 ± 1.3 | 0.06 |
| FEV1, L | 3.5 ± 0.7 | 2.5 ± 0.5 | 3.5 ± 0.7 | 2.3 ± 0.5 | 0.001 |
| FVC, L | 4.3 ± 0.9 | 3.4 ± 0.7 | 4.4 ± 0.9 | 3.3 ± 0.8 | 0.05 |
| FEV1/FVC × 100 | 80.7 ± 3.2 | 73.7 ± 9.7 | 79.9 ± 4.8 | 70.5 ± 10.4 | 0.001 |
| FEV1% predicted | 106.2 ± 6.1 | 76 ± 10.4 | 108.5 ± 7.4 | 74.9 ± 12.7 | 0.002 |
| FVC% predicted | 106.1 ± 6.2 | 83.1 ± 11.7 | 109.5 ± 8.9 | 85.3 ± 11.4 | 0.09 |
| Bronchodilator reversibility | 0 (0) | 1 (1.4) | 2 (2.9) | 8 (18.2) | 0.001 |
| TLC, L | 5.7 ± 0.5 | 6 ± 1.4 | 6 ± 1.2 | 6 ± 1.3 | 0.72 |
| DlCO, ml/min/mm Hg | 30.5 ± 2.9 | 23.2 ± 7 | 25.8 ± 6.6 | 23.3 ± 11.7 | 0.54 |
| Baseline COPD | 0 (0) | 19 (27.1) | 2 (2.9) | 18 (40.9) | 0.01 |
| Incident COPD | 2 (5) | 3 (4.3) | 3 (4.4) | 10 (22.7) | 0.01 |
| Days to Inc. COPD | 1,502 ± 775 | 1,101 ± 1021 | 1,506 ± 936 | 525 ± 618 | 0.001 |
| Age at Inc. COPD, yr | 52 ± 7.1 | 49 ± 5 | 49.3 ± 5.5 | 49.5 ± 2.8 | 0.69 |
| FEV% diff. | −4.7 ± 3 | −13.3 ± 15.8 | 8.9 ± 3.2 | 13.4 ± 10.3 | <0.0001 |
| FVC% diff. | −5.5 ± 4.2 | −10.9 ± 10 | 5.8 ± 4.7 | 5.1 ± 9.8 | <0.0001 |
| Ann. absolute FVC diff. | 0.1 | 0.1 | −0.1 | −0.1 | <0.0001 |
| Ann. decline rate, L | 0.06 ± 0.1 | 0.1 ± 0.1 | −0.09 ± 0.1 | −0.1 ± 0.1 | <0.0001 |
| Chron. bronchitis | 10 (25) | 29 (41.4) | 25 (36.8) | 18 (40.9) | 0.29 |
| mMRC score ≥ 2 | 10 (25) | 29 (41.4) | 19 (27.9) | 29 (67.4) | 0.004 |
| SGRQ total | 16.3 ± 17.6 | 24.2 ± 17.3 | 18.2 ± 15.8 | 28.8 ± 20.7 | 0.03 |
| SF-36 total | 118.5 ± 20.5 | 111.4 ± 21.3 | 117.3 ± 17.2 | 104.8 ± 20.5 | 0.04 |
| History of asthma | 7 (17.5) | 19 (27.5) | 15 (22.1) | 8 (18.6) | 0.79 |
Definition of abbreviations: Ann. = annual; BMI = body mass index; Chron. bronchitis = chronic bronchitis (self-reported cough and phlegm for at least 3 mo over the course of 2 consecutive years); COPD = chronic obstructive pulmonary disease; diff. = difference; HS = high school; Inc. = incident; LF = lung function; mMRC = modified Medical Research Council Dyspnea Questionnaire; ND = no decline; RD = rapid decline; SF-36 = 36-Item Short Form Questionnaire; SGRQ = St. George’s Respiratory Questionnaire.
Data are shown as n (%) or mean ± SD. Unless otherwise indicated, all variables shown are at baseline. Significant P values appear in bold. The P values shown are based on the Jonckheere Terpstra test for categorical variables and multivariate analysis of variance for continuous variables.
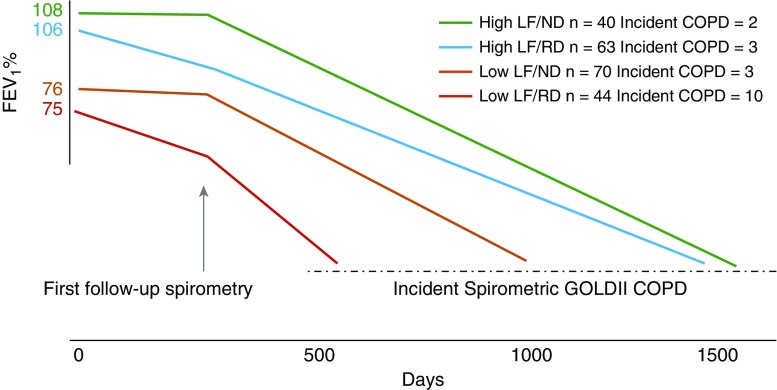
This study shows that among middle-aged heavy smokers, those with baseline low LF and RD are at the highest risk of developing COPD within 4 years of observation. Although the pathobiological pathways leading to COPD may already be initiated in those without disease at baseline, the clinical manifestations may not be visible at this stage. However, for those with incident COPD, the risk of developing COPD was extremely high, and the days to incident COPD was a third of the days for patients with high LF/ND (Figure 1). RD status can be determined with two spirometry readings spaced 12 months or more apart. Acknowledging that smoking cessation is of primary importance, these results have clinical relevance, in that subjects with baseline LF of FEV1 75% predicted had a 40% chance of being categorized in the RD category and, therefore, would benefit from serial screening spirometry measurements to determine LF trajectory. Furthermore, because subjects in the LF/RD group have the highest percentage of subjects with bronchodilator reversibility, LF measurements should be performed pre- and postbronchodilator administration to decrease variability of measurement. Self-reported history of doctor-diagnosed asthma was similar for all groups (Table 1). Low LF and rapid decline of 40 ml/year may help detect those smokers at highest risk for incident COPD and identify a subgroup of smokers that might benefit the most from intensive secondary preventative measures, such as smoking cessation strategies or avoidance of secondhand smoke exposure. Because the LSC is not representative of the general population, research efforts are needed to replicate this study in other cohorts. In addition, samples from subjects developing early COPD are needed to identify the pathobiological mechanisms responsible for baseline low LF and RD among younger individuals.
Figure 1.
Days to incident chronic obstructive pulmonary disease (COPD). Lung function (LF) as expressed by the FEV1% of predicted is determined at baseline, and the rate of decline by at least one postbronchodilator spirometry obtained 18 months apart. Incident COPD occurred earlier in subjects with low lung function at baseline, and the hazard ratio was higher in subjects with low LF at baseline and rapid rate of decline. See text for more details. GOLD = Global Initiative for Obstructive Lung Disease; ND = no decline; RD = rapid decline.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.201805-0861LE on August 16, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.de Marco R, Accordini S, Marcon A, Cerveri I, Antó JM, Gislason T, et al. European Community Respiratory Health Survey (ECRHS) Risk factors for chronic obstructive pulmonary disease in a European cohort of young adults. Am J Respir Crit Care Med. 2011;183:891–897. doi: 10.1164/rccm.201007-1125OC. [DOI] [PubMed] [Google Scholar]
- 2.Stang P, Lydick E, Silberman C, Kempel A, Keating ET. The prevalence of COPD: using smoking rates to estimate disease frequency in the general population. Chest. 2000;117(5) Suppl 2:354S–359S. doi: 10.1378/chest.117.5_suppl_2.354s. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Salcedo P, Divo M, Casanova C, Pinto-Plata V, de-Torres JP, Cote C, et al. Disease progression in young patients with COPD: rethinking the Fletcher and Peto model. Eur Respir J. 2014;44:324–331. doi: 10.1183/09031936.00208613. [DOI] [PubMed] [Google Scholar]
- 4.Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 5.Bruse S, Sood A, Petersen H, Liu Y, Leng S, Celedón JC, et al. New Mexican Hispanic smokers have lower odds of chronic obstructive pulmonary disease and less decline in lung function than non-Hispanic whites. Am J Respir Crit Care Med. 2011;184:1254–1260. doi: 10.1164/rccm.201103-0568OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sood A, Petersen H, Meek P, Tesfaigzi Y. Spirometry and health status worsen with weight gain in obese smokers but improve in normal-weight smokers. Am J Respir Crit Care Med. 2014;189:274–281. doi: 10.1164/rccm.201306-1060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez FJ, Han MK, Allinson JP, Barr RG, Boucher RC, Calverley PMA, et al. At the root: defining and halting progression of early chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197:1540–1551. doi: 10.1164/rccm.201710-2028PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178:332–338. doi: 10.1164/rccm.200712-1869OC. [DOI] [PubMed] [Google Scholar]