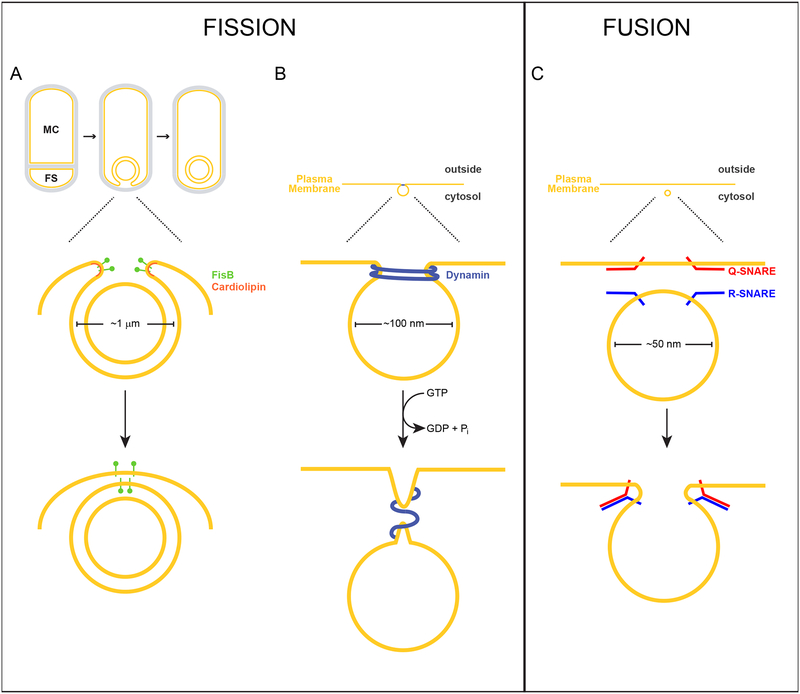
Figure 1. Membrane fission of the engulfing sporulation membrane mediated by FisB, or endocytic vesicles mediated by dynamin, and membrane fusion of synaptic vesicles mediated by SNARE complexes.
Above, scale drawings of a 1 μm diameter developing forespore of the bacterium Bacillus subtilis, 100 nm diameter endocytic vesicle, and a 50 nm diameter synaptic vesicle are depicted. Lipid bilayers are depicted in yellow; peptidoglycan is depicted in gray. (A) Sporulation in B. subtilis initiates with an asymmetric division event that divides the cell into two genetically identical compartments: the mother cell (MC) and forespore (FS). Next, the asymmetric septum curves as the mother cell engulfs the forespore. Eventually, the leading edges of the engulfing membrane undergo a membrane fission event, pinching off the forespore as a double membrane-bound organelle in the mother cell. FisB (green) localizes to the site of fission, where its extracellular domain interacts with the lipid cardiolipin (orange) which may be enriched along the concave (negatively curved) inner leaflet of the phospholipid bilayer at this site. (B) Dynamin (blue) assembles at the neck of an endocytic vesicle as it forms from the plasma membrane. GTP hydrolysis drives the constriction of dynamin, driving membrane scission. (C) During exocytosis, R-SNAREs anchored in the vesicular membrane interact with Q-SNARES on the target membrane to form a stable cis-SNARE complex that drives membrane fusion.