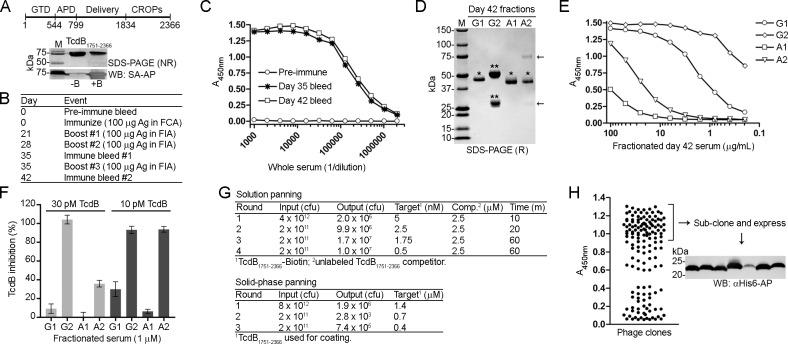
Fig 1. Isolation of high-affinity TcdB-binding VHHs.
(A, top) Schematic of TcdB. GTD, glucosyltransferase domain; APD, autoprotease domain, Delivery, delivery/receptor binding domain, CROPs, combined repetitive oligopeptides domain. (A, bottom) A 71.6 kDa fragment of C. difficile toxin B (TcdB1751-2366) was purified, biotinylated and analyzed by non-reducing (NR) SDS-PAGE and Western blot (WB) probed with streptavidin-AP (SA-AP). Approximately 1 μg of protein was loaded per lane. M, protein molecular weight marker; -B, unlabeled TcdB1751-2366; +B, biotinylated TcdB1751-2366. (B) TcdB1751-2366 was used as an antigen (Ag) for llama immunization with the schedule shown. FCA, Freund’s complete adjuvant; FIA, Freund’s incomplete adjuvant. (C) ELISA showing the binding response from pre-immune and immune llama sera collected on days 35 and 42 post immunization to coated TcdB1751-2366. (D) Serum from day 42 post immunization was fractionated using protein G and protein A affinity columns and analyzed by SDS-PAGE under reducing (R) conditions. G1, A1 and A2 fractions contain heavy-chain IgG (hcIgG, *) while the G2 fraction contains conventional IgG (cIgG, **). The arrows denote IgM heavy and light chains in the A2 fraction. (E) ELISA showing the binding response of fractionated day 42 serum to coated TcdB1751-2366. (F) Vero cell cytotoxicity assay demonstrating the effect of fractionated day 42 serum on TcdB inhibition, 72 h post addition of TcdB and polyclonal antibodies to Vero cell monolayers. TcdB was used at 10 and 30 pM and polyclonal fractionated sera at 1 μM. (G, top) Summary of solution-phase library panning titers using off-rate selection. In each round, the amount of target (biotinylated TcdB1751-2366) was reduced, the amount of competitor (“Comp”; unlabeled TcdB1751-2366) was held constant, and the incubation time of phage + target + competitor was increased. After incubation, the complex of phage and biotinylated TcdB1751-2366 was captured on streptavidin coated microtiter plates, washed and eluted. (G, bottom) Summary of solid-phase library panning titers using coated TcdB1751-2366. (H) Phage ELISA showing binding of phage displayed VHHs to coated TcdB1751-2366. VHHs on phage producing the highest ELISA signals were expressed, purified and characterized. The Western blot (WB) shows detection of purified VHHs with an α-His6-AP secondary antibody.