Figure 1.
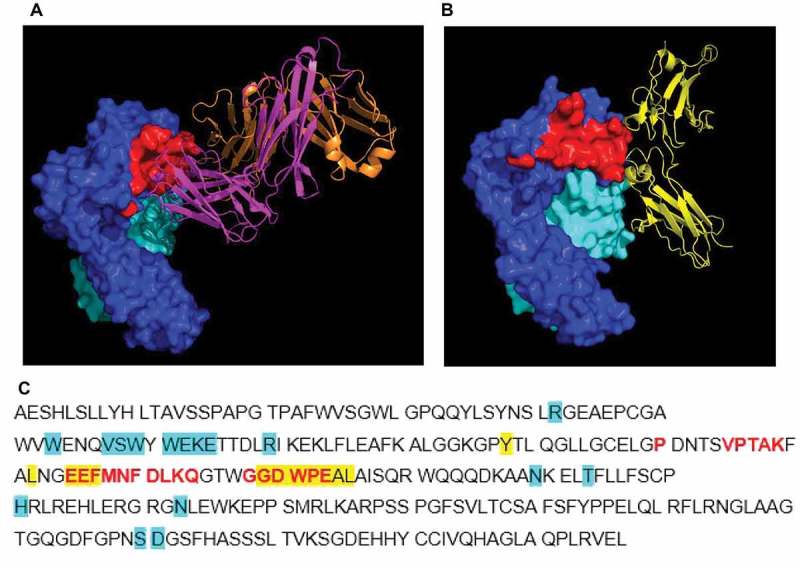
Crystal structure of complex of human FcRn (deglycosylated extracellular domain) and 1519.g57 Fab’. (A) The binding epitope of 1519.g57 Fab’ on FcRn α-chain is shown in red; (B) PDB 4N0U – human IgG Fc domain interacting with FcRn, demonstrating the overlapping epitope with that of 1519.g57Fab’,29 (C) FcRn α-chain sequence, showing residues involved in interaction with 1519.g57 Fab’ (in red). Residues involved in interaction between human FcRn and IgG (Fc domain) or albumin (as described by Oganesyan et al, 2014, highlighted in yellow or in blue, respectively) are also shown. The sequence of human FcRn α-chain ECD was taken from Swiss Prot (P55899-1); Residue F44, identified by Oganesyan et al.29 as interacting with albumin, is residue E44 in this sequence and in the sequence of Schmidt et al.30 Dark blue = FcRn α-chain; pale blue = β2 M; magenta = 1519.g57 heavy chain; orange = 1519.g57 light chain; yellow = IgG Fc domain; red = 1519.g57 Fab’ binding epitope on FcRn.
