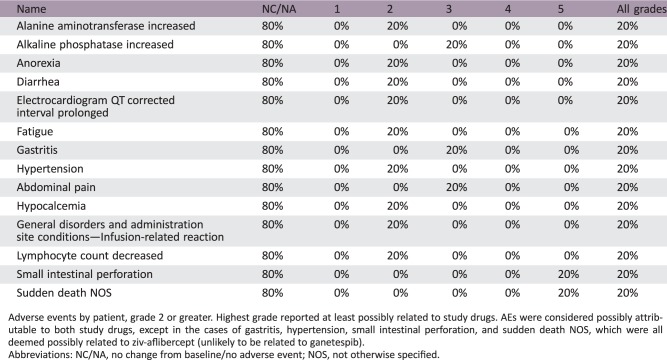
Adverse events by patient, grade 2 or greater. Highest grade reported at least possibly related to study drugs. AEs were considered possibly attributable to both study drugs, except in the cases of gastritis, hypertension, small intestinal perforation, and sudden death NOS, which were all deemed possibly related to ziv‐aflibercept (unlikely to be related to ganetespib).
Abbreviations: NC/NA, no change from baseline/no adverse event; NOS, not otherwise specified.