Table 1. Adverse events by patient.
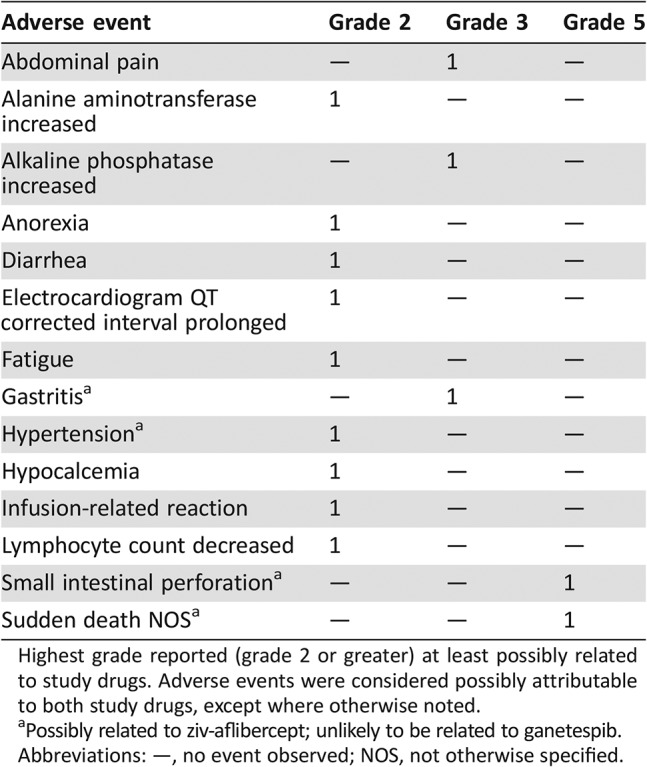
Highest grade reported (grade 2 or greater) at least possibly related to study drugs. Adverse events were considered possibly attributable to both study drugs, except where otherwise noted.
Possibly related to ziv‐aflibercept; unlikely to be related to ganetespib.
Abbreviations: —, no event observed; NOS, not otherwise specified.
