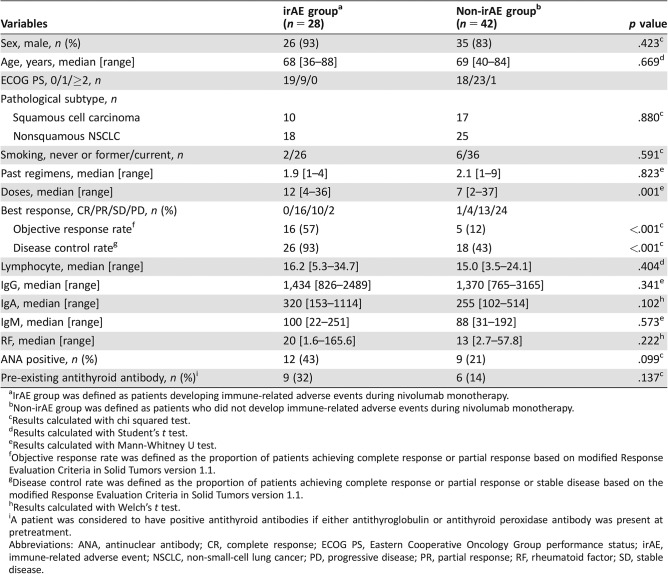
Table 3. Characteristics of patients in the irAE and non‐irAE groups (n = 70).
IrAE group was defined as patients developing immune‐related adverse events during nivolumab monotherapy.
Non‐irAE group was defined as patients who did not develop immune‐related adverse events during nivolumab monotherapy.
Results calculated with chi squared test.
Results calculated with Student's t test.
Results calculated with Mann‐Whitney U test.
Objective response rate was defined as the proportion of patients achieving complete response or partial response based on modified Response Evaluation Criteria in Solid Tumors version 1.1.
Disease control rate was defined as the proportion of patients achieving complete response or partial response or stable disease based on the modified Response Evaluation Criteria in Solid Tumors version 1.1.
Results calculated with Welch's t test.
A patient was considered to have positive antithyroid antibodies if either antithyroglobulin or antithyroid peroxidase antibody was present at pretreatment.
Abbreviations: ANA, antinuclear antibody; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; irAE, immune‐related adverse event; NSCLC, non‐small‐cell lung cancer; PD, progressive disease; PR, partial response; RF, rheumatoid factor; SD, stable disease.