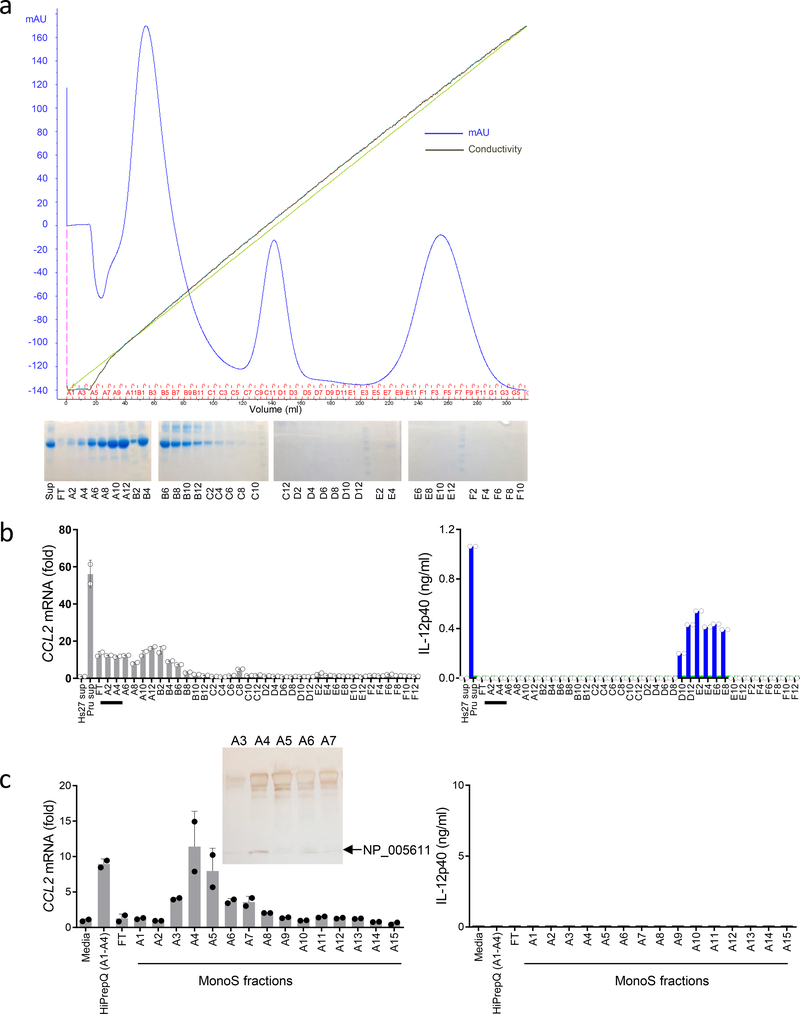
Figure 3.
Biochemical isolation of S100A11 as a CCL2-inducing molecule.
(a) Initial separation of cell culture supernatants collected from human fibroblasts infected with the Pru strain of T. gondii via HiPrepQ anion-exchange chromatography and (b) assay of individual fractions in duplicates for their ability to induce expression of CCL2 in THP-1 cells (left) and to stimulate IL-12p40 secretion by mouse splenocytes prepared from WT (blue bars) and Tlr11–/– (green bars) mice, right. mAU = milli-Absorbance Units. (c) Mono S cation-exchange chromatography of fractions A1-A4 from the HiPrepQ anion-exchange chromatography. Individual fractions were assayed in duplicates. Each symbol represents an individual experimental sample. The data shown are representative of (a-b) five and (c) three independent experiments, and error bars shown represent the mean ± SD. The inset shows an SDS-PAGE analysis of the Mono S cation-exchange chromatography fractions A3, A4, A5, A6, and A7, with the arrow indicating a band analyzed by mass-spectrometry that revealed the presence of the human S100A11 protein (NP_005611).