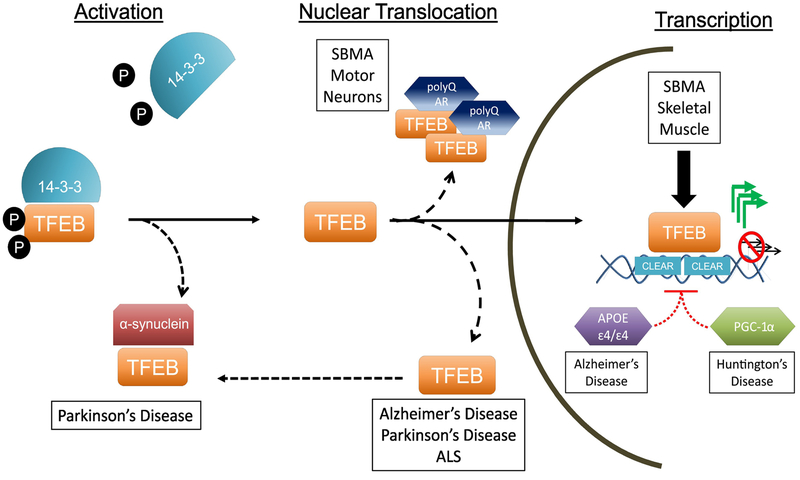
Figure 2. TFEB dysregulation in Neurodegenerative Disease.
TFEB function is tightly regulated at three distinct steps: its own activation, nuclear translocation, and transcription activity at its target genes. In its inactive form, phosphorylated TFEB interacts with 14–3–3 proteins, remaining sequestered in the cytosol. Upon activation (under conditions of lysosomal stress or mTOR inhibition), TFEB is dephosphorylated and dissociates from the 14–3–3 complex, unmasking its nuclear localization signal. TFEB can now translocate into the nucleus, driving transcription of the CLEAR network of target genes. Several of these steps have been reported to be dysfunctional in neurodegenerative disease, including TFEB sequestration (in PD), nuclear exclusion (in SBMA, AD, PD, and ALS) and transcription incompetence (in SBMA, AD, and HD). These observations indicate that TFEB dysregulation is a defining feature of neurodegenerative proteinopathies.