Fig. 1.
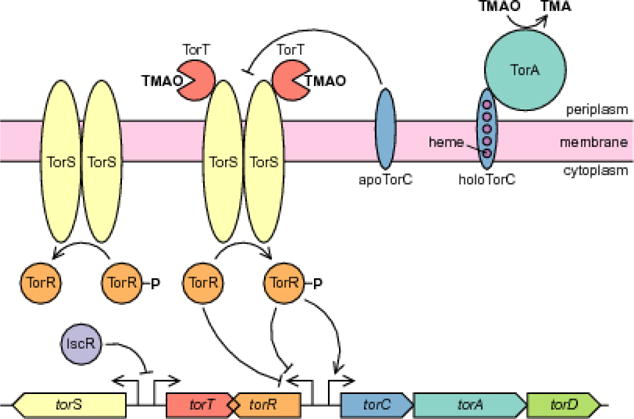
Regulation of the trimethylamine oxide (TMAO) respiratory system in E. coli. TorT detects TMAO in the periplasm and causes TorS to phosphorylate TorR. Phosphorylation of TorR requires a three-step intermolecular phosphorelay (not pictured) between the subunits of the TorS dimer before the final phosphotransfer to TorR (Jourlin et al. 1997; Ansaldi et al. 2001). Phosphorylated TorR activates transcription of the torCAD operon, which encodes the TMAO reductase complex TorCA. TorD is a chaperone for TorA maturation and export to the periplasm (Pommier et al. 1998; Ilbert et al. 2003; Jack et al. 2004). TorCA catalyzes the terminal reduction step of TMAO respiration, converting TMAO into trimethylamine (TMA). TorS dephosphorylates TorR-P when not interacting with TorT-TMAO, thereby deactivating torCAD transcription. Expression of torR is negatively autoregulated by both phosphorylated and unphosphorylated TorR (Ansaldi et al. 2000). TorC also participates in negative autoregulation, with apoTorC (the immature form lacking its heme cofactors) inhibiting TorS and preventing TorR phosphorylation. Transcription of torS and torT is repressed by the transcription factor IscR, which is more abundant in aerobic conditions
