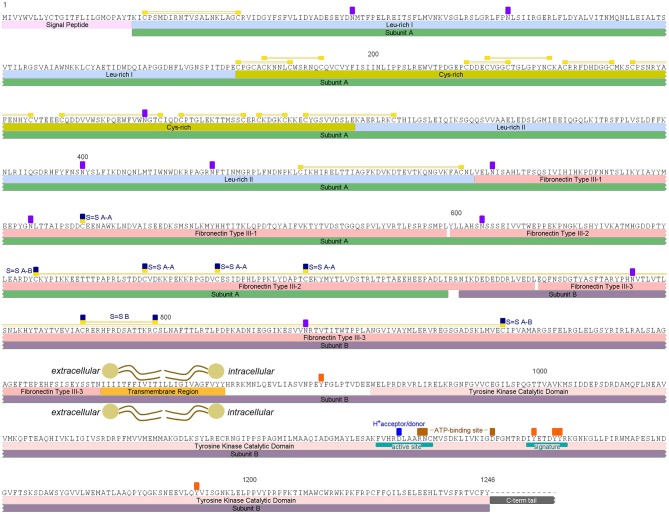
Figure 1.
Rhopr-IR putative amino acid sequence and predicted conserved features and domains. Rhopr-IR monomer subunits A and B are highlighted by green and purple boxes, respectively. Signal peptide is highlighted in light pink, followed by Leu-rich domains highlighted in blue and a Cys-rich domain highlighted in dark yellow. Disulfide bonds are identified by yellow boxes over Cys residues, connected by two yellow lines. FnIII domains are indicated by pink boxes. The Cys residues in the subunit A that form disulfide bonds with Cys residues in the other subunit A of the active receptor dimer are indicated by yellow/dark blue boxes over the residues (S = S A-A). The two Cys residues that form the disulfide bond between subunits A and B of the monomer, are indicated by yellow/dark blue boxes over the residues (S-S A-B) connected by two yellow lines. The transmembrane region is highlighted by an orange box, and the Tyr kinase catalytic domain by a pink box. The active site, F-V-H-R-D-L-A-A-R-N-C (1097–1107), containing a proton donor/acceptor, and the signature that characterizes receptors Tyr kinase class II, D-I-Y-E-T-D-Y-Y-R (1125–1133), are indicated with teal boxes within the catalytic domain. ATP-binding sites between the active site and the receptor signature are indicated with brown boxes over the residues. Potentially phosphorylated Tyr residues in the intracellular region and potentially glycosylated Asn residues in the extracellular portion, are indicated by orange and purple boxes, respectively.