Abstract
Angiogenesis plays a critical role in neural repair following ischemic stroke. Therapeutic angiogenesis contributes to neurological functional recovery after cerebral infarction. Nerve growth factor (NGF) has been reported as a neurotrophic factor. However, the angiogenic efficacy of NGF in cerebral ischemia remains unclear. In this study, we investigated the effect of NGF on angiogenesis in the ischemic penumbra and neurological outcome in a rat model of middle cerebral artery occlusion (MCAO). Our results demonstrate that the intranasal administration of NGF improves neurological outcome and reduces infarct volume on day 7 after MCAO in rats. Treatment with NGF promoted angiogenesis in the peri-infarct region, increased the serum levels of VEGF and SDF-1 protein, and elevated the number of circulating endothelial progenitor cells (EPCs) on day 4 after MCAO. In addition, NGF enhanced capillary-like tube formation of rat brain microvascular endothelial cells in vitro, further confirming its angiogenic effect. Furthermore, the neuroprotective and angiogenic effects of NGF can be significantly attenuated by the phosphatidylinositide 3-kinase (PI3K)/Akt pathway antagonist LY294002. Our results indicate that NGF-enhanced angiogenesis contributes to neurological functional recovery after ischemic stroke, which may occur partly via activation of the PI3K/Akt signaling pathway. This study provides novel experimental evidence for the angiogenic role of NGF in treating ischemic stroke.
Keywords: Nerve growth factor, angiogenesis, ischemic stroke, PI3K/Akt signaling pathway
Introduction
Stroke is one of the major causes of death or severe long-term disability worldwide. Effective drugs for treatment of acute ischemic stroke remain quite limited. Although treatment with intravenous recombinant tissue-type plasminogen activator, the only FDA-approved drug treatment for acute ischemic stroke, can effectively reduce disability after acute ischemic stroke, it must be administered within 4.5 hours after the onset of stroke. Therefore, pharmacological therapy aimed to promote post-stroke brain repair has drawn much attention in the last decade. Angiogenesis plays a critical role in neuroplasticity following ischemic stroke. Acute ischemic stroke induces angiogenesis, and vascular remodeling participates in the complex process of brain repair after cerebral ischemia, including resorting of cerebral blood flow, supplying neurotrophic factors for neural stem cells, and improving synaptic plasticity. Clinical evidence reveals that there is a significant increase in the number of microvessels in the penumbra of patients with cerebral infarction, and higher microvessel density may be related to longer survival time after stroke [1]. In agreement with the results of other research groups, our previous studies showed that treatment with exogenous angiogenic factors contributes to the recovery of neurological function in animal models of cerebral ischemia [2-5]. Thus, therapeutic angiogenesis may represent a promising strategy for the treatment of ischemic stroke.
Nerve growth factor (NGF) is the first identified member of the neurotrophic family. NGF binds to its specific tropomyosin-related kinase (TrkA) receptor and plays crucial roles in the growth, differentiation, and survival of distinct neurons in the peripheral and central nervous systems. Although NGF was initially discovered as a neurotrophic factor, increasing evidence has shown that NGF also exerts actions on both physiological and pathologic processes of angiogenesis [6-9]. It is well known that cerebral ischemia can upregulate the expression of many proangiogenic factors in the ischemic area. A recent study revealed that NGF could enhance the secretion of vascular endothelial growth factor (VEGF) and promote cell proliferation via the phosphatidylinositide 3-kinase (PI3K)/Akt pathway in Müller cells [10]. Additionally, NGF could promote migration and proliferation of human choroidal endothelial cells [11], as well as enhance endothelial progenitor cell (EPC)-mediated angiogenic responses [12].
Experimental studies have documented that the use of NGF increases capillary density in animal models of ischemic diseases including hindlimb ischemia and myocardial infarction [7-9,13]. However, the efficacy of NGF in promoting post-ischemic stroke angiogenesis has not been well elucidated. In this study, we investigated the hypothesis that the administration of NGF could promote angiogenesis in the ischemic penumbra and improve neurological outcome in a rat model of ischemic stroke. Moreover, the underlying molecular mechanisms of NGF-enhanced angiogenesis on post-ischemic recovery were also explored.
Materials and methods
Experimental animals
A total of 72 male Sprague-Dawley rats weighing 200-250 g were used in this study. All rats were housed in an air-conditioned room with a 12:12 h light/dark cycle and were raised with free access to food and water. All experimental procedures were approved by the local Institutional Animal Care and Use Committee and in accordance with guidelines for animal use. We made significant effort to minimize the animals’ suffering during the experiments.
Middle cerebral artery occlusion (MCAO)
The model rats were made by MCAO according to previously described procedures [5]. Briefly, rats were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneally), and then a ventral midline incision was made and the right common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were gently exposed. A 4.0 monofilament nylon suture with a tip diameter of 0.28 mm (Cinontech Co., Ltd., Beijing, China) was carefully inserted through the right ECA into the right ICA about 18.0 ± 1.0 mm from the carotid artery bifurcation, and then gently advanced to occlude the origin of the right middle cerebral artery. After 2 h of MCAO, the suture was carefully withdrawn and reperfusion was confirmed by laser Doppler. Heat lamps were used to maintain body temperature at 37 ± 0.5°C during the operative procedures.
Experimental groups
All rats were randomly assigned to the NGF group, vehicle group, or NGF + LY294002 group. Rats in the NGF group or vehicle group were intranasally administered with NGF (60 μg/kg/d, NOBEX; Sinobioway Biomedicine Co., Ltd., Xiamen, Fujian, China) or the same volume of 0.9% saline daily for 6 consecutive days starting 2 h after MCAO. Rats in the NGF + LY294002 group were pretreated with LY294002 (10 μL, 10 mM in 100% DMSO, CST, USA) by right intraventricular injection 30 min before MCAO, and then intranasally administered with NGF (60 μg/kg/d) daily for 6 consecutive days starting 2 h after MCAO.
Intranasal administration
After anesthesia, rats were placed in a supine position. NGF was dissolved in 0.9% saline and intranasally delivered at doses of 60 µg/kg/d, 5 µl at a time, via alternating nostrils with an interval of 5 min between dosings. Rats of the vehicle group received 0.9% saline in the same manner.
Bromodeoxyuridine administration
5’-bromo-2’-deoxyuridine (BrdU) was used to label proliferative cells. Rats of each group were given intraperitoneal injection of BrdU (50 mg/kg, Sigma-Aldrich, St. Louis, MO, USA) twice daily for 3 consecutive days beginning on day 4 after MCAO.
Neurological function assessment
Bederson scores were used to evaluate neurological function in all rats on days 1, 4, and 7 after MCAO, as previously described [14]: 0, extension of both forelimbs toward the floor and no other neurological deficits; 1, contralateral forelimb flexion and no other neurological deficits; 2, reduced resistance to lateral push without circling; and 3, consistent circling to the paretic side.
Infarct volume measurement
Infarct volume was measured using 2, 3, 5-triphenyltetrazolium chloride (TTC, Sigma-Aldrich, USA) staining on day 7 after MCAO. In brief, rats were anesthetized, and then brains were rapidly removed and cut into five 2 mm thick coronal sections. The sections were incubated with 2% TTC at 37°C for 30 min, and then immersed in 4% paraformaldehyde at 4°C overnight. The TTC-stained sections were photographed and analyzed by a computer imaging analysis system. The relative infarct volume was presented as the percentage of the contralateral hemisphere and analyzed using ImageJ 1.46 software (Image J, NIH, MD, USA).
Enzyme-linked immunosorbent assay (ELISA)
Rats were deeply anesthetized and blood samples were obtained from the abdominal aorta on day 4 after MCAO. After centrifugation at 3,000 rpm for 15 min, the supernatant was collected. The concentrations of VEGF and stromal cell-derived factor 1 (SDF-1) were measured using a rat VEGF ELISA kit (RRV00, R&D Systems, USA) and a rat SDF-1 ELISA kit (KA3681, Abnova, Taiwan), according to the manufacturer’s instructions.
Flow cytometry
The number of circulating EPCs from peripheral blood was measured by flow cytometry. Briefly, peripheral blood mononuclear cells were separated from fresh blood samples on day 4 after MCAO, and then subjected to density gradient centrifugation at 300 × g for 20 min at room temperature, washed three times with 0.01 M phosphate-buffered saline (PBS), and then stained with anti-rat CD34-PE (Abcam, USA) and anti-rat KDR-FITC (Abcam, USA) for 30 min at room temperature in the dark. Mouse IgG1-PE isotype (Abcam, USA) was used as a control antibody. Samples were analyzed by flow cytometry using the Novocyte flow cytometer (2060R, ACEA Biosciences, USA). Data were analyzed using the FlowJo software.
Immunofluorescence staining
Rats were anesthetized and transcardially perfused with heparinized saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB). The rat brains were taken out immediately, postfixed for 6 h, sequentially immersed in 15%, 20%, 30% sucrose/PB solution, and then cut as 10 μm coronal sections (VT 2800N; Leica, Heidelberg, Germany). The brain sections were frozen at -80°C.
Immunofluorescence staining was performed as previously described [5,15]. Frozen coronal sections were blocked in 10% normal donkey serum for 1 h and then incubated with primary antibodies [mouse monoclonal anti-BrdU (1:1000, Sigma-Aldrich, USA), rabbit polyclonal anti-laminin (1:400, Sigma-Aldrich, USA)] at 4°C overnight. The secondary antibodies Alexa Fluor 488 (1:200, Invitrogen, USA) and Alexa Fluor 594 (1:200, Invitrogen, USA) were applied for 1 h at room temperature. For immunofluorescent staining for BrdU, we pretreated the brain sections using 2 NHCl at 37°C for 30 min, and then rinsed them in 0.1 M boric acid (pH 8.5) at room temperature for 10 min before they were incubated with the blocking solution. Negative control sections were incubated with 0.01 M PBS as a substitute for the primary antibody. Images were captured with a fluorescence microscope (DM600b, Leica, Germany) and analyzed using ImageJ.
Western blot analysis
Fresh brain tissues were lysed with lysis buffer. Protein concentration was determined using standard Bradford assay. Equal amounts of protein from each experimental group were loaded onto 10% SDS-polyacrylamide gels and then transferred to PVDF membranes. After being blocked with 4% non-fat milk in TBS-T buffer for 2 h at room temperature, membranes were incubated with rabbit polyclonal anti-Akt antibody (1:5000, Abcam) or rabbit polyclonal anti-p-Akt antibody (1:1000, Cell Signaling Technology) at 4°C overnight. Rabbit monoclonal anti-β-actin (1:1000, Cell Signaling Technology) was used as a loading control. After being washed with TBS-T buffer, the membranes were incubated with horseradish peroxidase-conjugated anti-rabbit IgG (1:2000, Cell Signaling Technology) at room temperature for 1 h. Immunoreactive bands were visualized using the enhanced chemiluminescence method. The blots were semiquantified using the Bio-Rad Image LabTM Version 3.0 software (Bio-Rad, USA).
Capillary-like tube formation assay
To examine the angiogenic action of NGF in vitro, a capillary-like tube formation assay was performed using rat brain-derived microendothelial cells (rBMECs). Growth factor-reduced Matrigel (BD Biosciences, USA) was plated onto 24-well plates and incubated at 37°C for 30 min. The cultured 2nd passage rBMECs were seeded at 5 × 104 cells/well in Dulbecco’s Modified Eagle Medium (DMEM), DMEM containing NGF, or DMEM containing NGF + LY294002. Forty-eight hours later, the medium was removed. The cells were washed with 0.01 M PBS, fixed with 4% paraformaldehyde, and then stained with Calcein AM (Invitrogen, Carlsbad, CA). The branch length of capillary-like tube structures was imaged and measured at 5 random areas of each well using a microscope with 20 × magnification (Leica DMI4000B, Germany). All assays were carried out in triplicate.
Statistical analysis
Quantitative data are expressed as means ± SD. Differences between two groups were examined using Student’s t-test and statistical comparisons between multiple groups were made by one-way ANOVA followed by least significant differences test. All numerical data were analyzed with SPSS 18.0 for Windows. P < 0.05 was considered statistically significant.
Results
NGF improves neurological functional outcome after ischemic stroke
We measured neurological function using modified Bederson scores on days 1, 4, and 7 after MCAO in all rats. Compared with those in the vehicle group, rats in the NGF-treated group showed better neurological recovery on days 1, 4, and 7 after MCAO, while rats in the NGF + LY294002 group exhibited worse neurological outcome than those of the NGF group at the same point after MCAO (Figure 1). These data suggest that treatment with NGF significantly enhanced functional recovery after ischemic stroke, which occurred partly via PI3K/Akt signaling.
Figure 1.
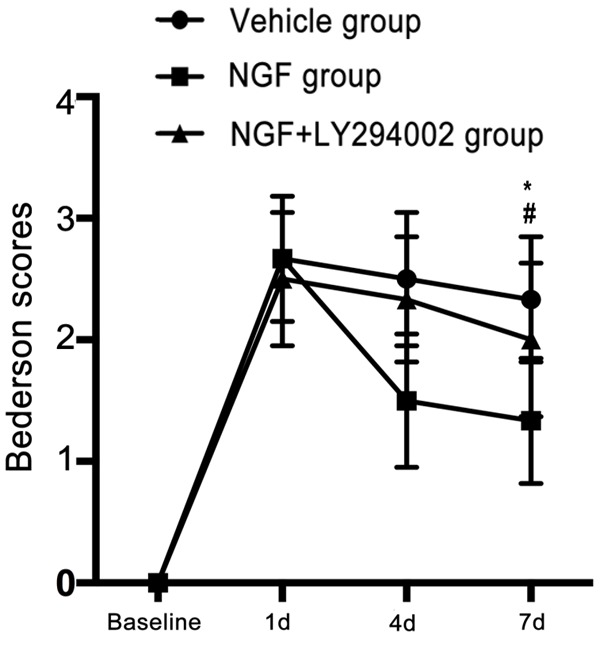
NGF improves neurological functional outcome after ischemic stroke in rats. Graphs show the Bederson scores for rats in the vehicle, NGF, and NGF + LY294002 groups on day 7 after MCAO. Significant improvements in neurological function were observed in the NGF group compared with the vehicle group. *P < 0.05 versus vehicle group, and #P < 0.05 versus NGF group.
NGF reduces cerebral infarct volume after ischemic stroke
To further examine whether intranasal administration of NGF could improve neurological outcome after ischemic stroke, we used TTC staining to detect cerebral infarct volume. NGF-treated rats had smaller cerebral infarct volumes than vehicle-treated rats on day 7 after MCAO. However, the cerebral infarct volume of NGF + LY294002-treated rats was significantly larger compared to that of the NGF-treated rats at the same time point after MCAO (Figure 2). These results indicate that treatment with NGF significantly reduced cerebral infarct volume in the MCAO rats, and that blocking PI3K/Akt signaling could significantly decrease the neuroprotective effect of NGF in the setting of ischemic stroke.
Figure 2.
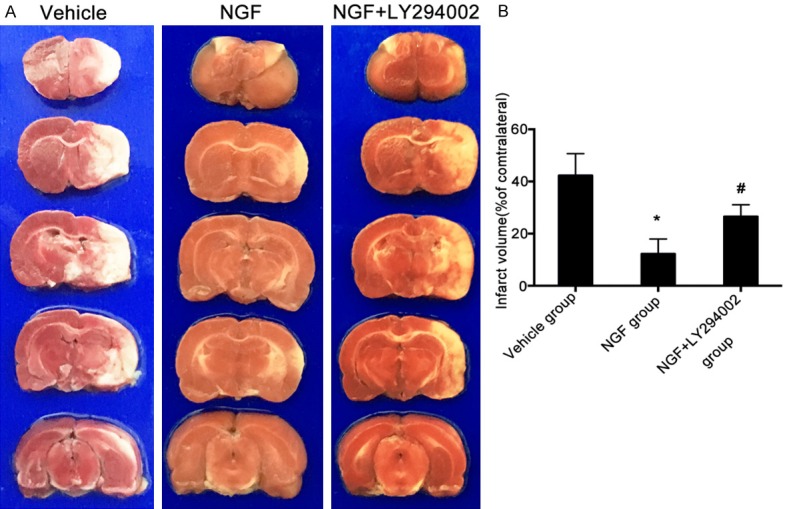
NGF reduces cerebral infarct volume after ischemic stroke in rats. Representative images of brain sections stained by TTC (A) and quantification of infarct volume (B) in rats of the vehicle, NGF, and NGF + LY294002 groups on day 7 after MCAO. Data are given as the mean ± SD (n = 6 animals per group). Mean infarct volume of rats in the NGF group was significantly reduced compared to rats in the vehicle and NGF + LY294002 groups. *P < 0.05 versus vehicle group, and #P < 0.05 versus NGF group.
NGF upregulates serum levels of VEGF and SDF-1 in MCAO rats
To investigate whether intranasal administration of NGF increases the expression of angiogenic factors after ischemic stroke, serum VEGF and SDF-1 levels were detected by ELISA on day 4 after MCAO. Treatment with NGF significantly increased serum VEGF and SDF-1 levels compared to the vehicle group (Figure 3). Rats of the NGF + LY294002 group showed significantly reduced levels of VEGF compared to the NGF group (Figure 3). However, there was no difference in SDF-1 level between the NGF treatment group and NGF + LY294002 treatment group. These results suggest that intranasal delivery of NGF increased the serum levels of angiogenic factors following ischemic stroke, which occurred partly through the PI3K/Akt signaling pathway.
Figure 3.
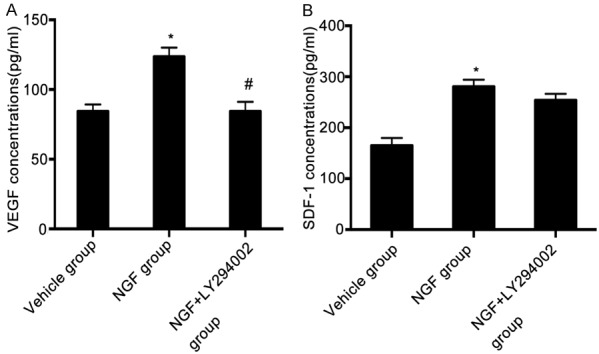
NGF upregulates the serum level of VEGF and SDF-1 after ischemic stroke in rats. Blood samples were obtained from the abdominal aorta and the supernatant was collected. Serum levels of VEGF protein (A) or SDF-1 protein (B) in rats of the vehicle, NGF, and NGF + LY294002 groups on day 4 after MCAO, measured by ELISA. Rats from the NGF + LY294002 group showed significantly reduced VEGF expression compared to the NGF group. *P < 0.05 versus vehicle group, and #P < 0.05 versus NGF group.
NGF enhances the number of circulating EPCs in MCAO rats
To determine whether intranasal delivery of NGF mobilizes EPCs from bone marrow into peripheral blood after ischemic stroke, we measured the number of circulating EPCs in peripheral blood from MCAO rats by flow cytometry. In this analysis, double immunofluorescence staining of CD34+ and KDR+ cells was used to identify bone marrow-derived EPCs. On day 4 after MCAO, the number of circulating CD34+/KDR+ cells was significantly higher in the NGF-treated group than in the vehicle-treated group (Figure 4). Treatment with NGF + LY294002 decreased the number of circulating CD34+/KDR+ cells compared with NGF treatment alone (Figure 4). These results show that intranasal administration of NGF can enhance mobilization of EPCs, which occurs partly by activation of the PI3K/Akt signaling pathway.
Figure 4.

NGF increases the number of circulating EPCs in MCAO rats. Peripheral blood mononuclear cells were stained with anti-rat CD34-PE and anti-rat KDR-FITC, and double immunofluorescence staining of peripheral blood CD34+/KDR+ cells was used to identify EPCs. Representative images of the number of circulating EPCs measured by flow cytometry in the vehicle group (A), NGF group (B), and NGF + LY294002 group (C). Quantification of circulating EPCs in the peri-infarct region on day 4 after MCAO for each group (D). *P < 0.05 versus vehicle group, and #P < 0.05 versus NGF group.
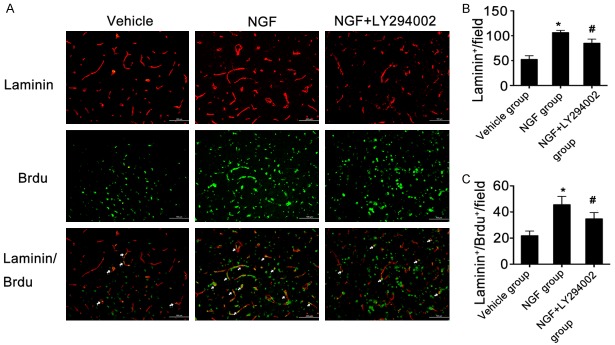
NGF promotes angiogenesis in the peri-infarct region after ischemic stroke
Immunofluorescence staining was performed to investigate the effect of intranasal administration of NGF on angiogenesis after ischemic stroke. Intranasal NGF treatment significantly increased microvessel density in the peri-infarct region, as compared with the vehicle treatment group on day 7 after MCAO (Figure 5). A lower microvessel density was observed in the peri-infarct region of NGF + LY294002-treated rats compared to NGF-treated rats at the same point after MCAO (Figure 5).
Figure 5.
NGF promotes angiogenesis in the peri-infarct region after ischemic stroke in rats. Laminin-immunopositive cells (red), BrdU-immunopositive cells (green), double-immunofluorescent labeling with antibodies against BrdU and laminin (yellow; indicated by the arrow). (A) Representative images of microvessel density and of BrdU+/laminin+ cells (arrow) in the peri-infarct area in rats of the vehicle, NGF, and NGF + LY294002 groups on day 7 after MCAO. Quantification of microvessel density (B) or the number of BrdU+/laminin+ cells (C) in the peri-infarct area on day 7 after MCAO for each group. Images were captured with a fluorescence microscope (DM600b, Leica, Germany). *P < 0.05 versus vehicle group, and #P < 0.05 versus NGF group. Scale bar, 100 μm.
To further verify the efficacy of intranasal administration of NGF in promoting angiogenesis in the rat model of MCAO, double immunofluorescence staining of both BrdU and laminin was used to identify newly formed vascular endothelial cells. We found that the number of BrdU+/laminin+ cells in the peri-infarct region was significantly higher in NGF-treated rats than that of vehicle-treated rats (Figure 5). Moreover, the angiogenic effect of NGF was significantly inhibited by the PI3K/Akt pathway antagonist LY294002 (Figure 5). These data suggest that treatment with NGF promotes angiogenesis in the peri-infarct region after ischemic stroke, which occurs partly via activation of the PI3K/Akt signaling pathway.
NGF increases Akt phosphorylation in the peri-infarct region after ischemic stroke
To further examine whether NGF exerts its angiogenic action on brain repair via activation of the PI3K/Akt signaling pathway, we monitored the expression of p-Akt in the peri-infarct region by Western analysis. Treatment with NGF significantly upregulated the expression of p-Akt compared to vehicle-treated rats on day 7 after MCAO (Figure 6). However, pre-treatment with LY294002 followed by NGF administration resulted in a significant reduction of p-Akt expression compared to treatment with NGF alone at the same point after MCAO (Figure 6). These results further confirm that the PI3K/Akt signaling pathway plays a critical role in NGF-enhanced angiogenesis after ischemic stroke.
Figure 6.
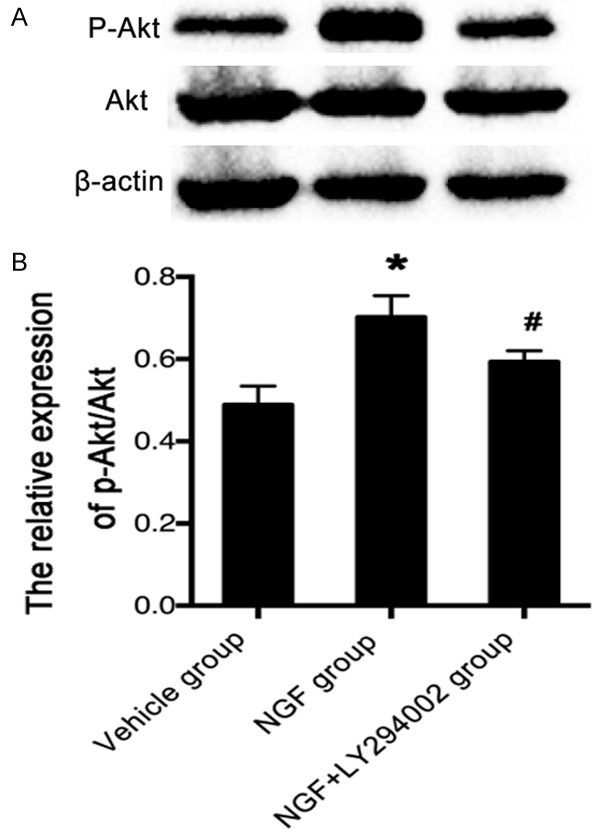
NGF stimulates p-Akt in the peri-infarct region after ischemic stroke in rats. A. Representative Western blots of p-Akt and Akt expression on day 7 after MCAO in rats of the vehicle, NGF, and NGF + LY294002 groups. B. Bar graph shows the relative expression of p-Akt/Akt in rats of the vehicle, NGF, and NGF + LY294002 groups on day 7 after MCAO. NGF significantly increased the expression of p-Akt, and pre-treatment with LY294002 followed by NGF administration resulted in a reduction of p-Akt expression. β-actin is used as an internal standard. *P < 0.05 versus vehicle group, and #P < 0.05 versus NGF group.
NGF stimulates capillary-like tube formation in vitro
We performed a capillary tube formation assay to confirm the efficacy of NGF in enhancing angiogenesis, using rat cerebral rBMECs. First, we incubated rBMECs with varying concentrations of NGF and established that the most effective concentration for tube formation was 500 μg/ml. Administration of NGF significantly induced capillary-like tube formation of rBMECs on growth factor-reduced matrigel when compared with the control treatment group. NGF-enhanced capillary-like tube formation was significantly inhibited when rBMECs were pre-treated with LY294002 (30 μmol/ml) followed by administration of NGF (Figure 7). These data indicate that NGF increases angiogenesis in vitro, and that the PI3K/Akt signaling pathway plays a crucial role in the angiogenic effect of NGF.
Figure 7.
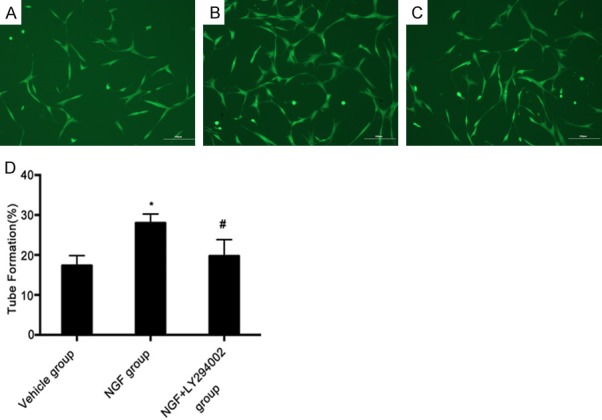
NGF stimulates capillary-like tube formation in vitro. Representative images of capillary-like tube formation of rat brain-derived micro-endothelial cells treated with vehicle (A), NGF (B), or NGF + LY294002 (C). NGF promoted capillary-like tube formation of rat cerebral rBMECs at 500 μg/ml. Bar graph showing quantification of the length of capillary-like tube formation for each group (D). *P < 0.05 versus vehicle group, and #P < 0.05 versus NGF group. Scale bar, 100 μm.
Discussion
In this study, we found that intranasal administration of NGF improved neurological functional outcome and reduced infarct volume in a rat model of cerebral ischemia. Treatment with NGF promoted angiogenesis in the peri-infarct region, increased the serum levels of VEGF and SDF-1 protein, and elevated the number of circulating EPCs after ischemic stroke. NGF promoted capillary-like tube formation of rBMECs in vitro, further confirming its angiogenic effect. Furthermore, the neuroprotective and angiogenic effects of NGF can be significantly attenuated by a PI3K/Akt pathway antagonist, LY294002. Altogether, our results indicate that NGF-enhanced angiogenesis may contribute to neurological functional recovery after ischemic stroke, which may partly occur via activation of the PI3K/Akt signaling pathway.
It is well established that angiogenesis plays an essential role in brain repair following ischemic stroke. The penumbra is the most critical therapeutic target in the acute phase of ischemic stroke. Our previous studies and those of others have shown that therapeutic angiogenesis promotes microvascular endothelial cell growth and improves tissue perfusion in the peri-infarct area, which benefits regeneration by supplying oxygen and energy substrates to the ischemic brain tissue [2,3,15]. In the past decade, most studies about the neuroprotective action of NGF have only focused on its role in enhancing the growth, differentiation, and survival of neurons in the peripheral and central nervous systems [16,17]. Recent studies have shown the effect of NGF on angiogenesis in animal models of ischemic hindlimb and myocardial ischemia [7-9,13]. However, whether treatment of NGF could improve angiogenesis following cerebral ischemia remained unclear. Our study shows that NGF has angiogenic properties in the setting of cerebral ischemia. We found that NGF treatment significantly increased microvessel density and newly formed vascular endothelial cells in the peri-infarct region in MCAO rats.
The question arises, what is the cellular basis of the angiogenic action of NGF on post-stroke angiogenesis? Increasing evidence suggests that angiogenesis and neurogenesis are interrelated neurorestorative mechanisms, i.e., angiogenesis is directly linked to neurogenesis following ischemic stroke [18,19]. Consistent with findings of other researchers, our previous studies also confirmed that migrating neural progenitor cells (NPCs) are closely associated with newly formed microvessels within the neurovascular niche in a rat model of focal cerebral infarction [5,15]. In the present study, we observed an increased number of nascent vascular endothelial cells labeled as BrdU+/laminin+ in the ischemic penumbra in NGF-treated MCAO rats. Together with the results of a recent study showing that NGF treatment enhances neural stem cell survival in the striatum and improves functional outcome after experimental cerebral ischemia [20], we postulate that NGF-stimulated newly formed microvascular endothelial cells might secrete various growth factors and chemokines to provide neurotrophic support to NPCs, fostering the survival of NPCs in the neurovascular niche and directing NPC migration toward the ischemic brain region after stroke. Therefore, it is reasonable to expect that the interaction of NGF-enhanced angiogenesis and neurogenesis may benefit neurological outcome after ischemic stroke.
As the processes underling ischemia-triggered angiogenic remodeling are very complex, we investigated the underlying molecular mechanisms of NGF’s angiogenic action on ischemic stroke. It is known that increased expression of angiogenic factors is seen in the ischemic penumbra within hours of stroke onset and lasts up to several days following cerebral ischemia [20,21]. Moreover, the elevated expression of angiogenic factors correlates with the appearance of newly generated vessels in the peri-infarct area [22]. Among angiogenic factors, VEGF is known as the most powerful mitogen for endothelial cells, and SDF-1 is crucial in EPC mobilization and homing to ischemic sites. Our study showed that there was an elevated level of serum VEGF on day 4 after MCAO in NGF-treated rats, indicating that exogenous NGF may directly exert angiogenic action by upregulating the expression of VEGF. Furthermore, EPCs have important roles in angiogenesis and the repair of injured endothelium. The mobilization, recruitment, and homing of EPCs require the participation of multiple angiogenic factors, including VEGF and SDF-1 [23-25]. VEGF was reported to promote corneal neovascularization by mobilizing EPCs [23]. Under ischemic conditions, the expression of SDF-1 and CXC chemokine receptor 4 (CXCR4) increased in ischemic tissues, promoting homing of EPCs to the sites of ischemia [24,25]. These findings suggest that interactions between angiogenic factors and EPCs contribute to promoting angiogenesis after ischemic injury. In agreement with the elevated serum expression of VEGF, our study also showed that there was a significant increase in the number of circulating EPCs in NGF-treated rats after ischemic stroke. Taken together, we conclude that the NGF-enhanced upregulation of VEGF expression may further mobilize bone marrow-derived EPCs into peripheral blood, which in turn fosters the angiogenic effect of NGF in ischemic stroke.
Accumulating evidence from a number of studies has shown that the PI3K/Akt signaling pathway plays a key role in angiogenesis after ischemic injury. The expression of multiple angiogenic molecules, such as VEGF and SDF-1, may be upregulated by the PI3K/Akt signaling pathway [26]. CXCR4-mediated proliferation and migration of EPCs after hypoxia involves activation of the PI3K/Akt signaling pathway, and downregulation of CXCR4 could inhibit angiogenesis through downregulation of the PI3K/Akt pathway [27,28]. The SDF-1/CXCR4 axis also can promote VEGF-mediated angiogenesis through the PI3K/Akt signaling pathway [4]. These findings indicate that the PI3K/Akt signaling pathway is involved in the angiogenic process after ischemic stroke. NGF and its receptor, TrkA, are able to stimulate the expression of VEGF and enhance the survival and growth of endothelial cells [29]. Other studies have revealed that activation of TrkA at the cell surface activates multiple signaling pathways involving PI3K, leading to activation of multiple downstream effectors including Akt, which is essential for VEGF-induced angiogenesis and endothelial cell survival [30,31]. Ahluwalia and colleagues also found that NGF’s angiogenic action on rat endothelial cells was mediated via PI3K/Akt signaling [32]. These findings support the notion that the PI3K/Akt signaling is involved in the angiogenic actions of NGF in vitro. Our findings showed that the upregulation of serum VEGF expression was significantly inhibited and the increase in circulating EPCs was blocked by LY294002, an inhibitor of PI3K/Akt signaling. Collectively, these data suggest that NGF promotes post-stroke angiogenesis in part by activating PI3K/Akt signaling. Studies have shown that miR-221 is essential for angiogenesis by regulating PI3K signaling; however, we did not explore related genes [33].
The blood-brain barrier is a highly selective semipermeable membrane barrier, which limits or prevents the entry of therapeutic drugs in the treatment of stroke. In light of this, a crucial question in considering neurotrophic factors as therapeutic candidates is how to promote entry into the central nervous system. In the last decade, intranasal administration has been proven to be a non-invasive and effective method for delivery of large molecular weight therapeutics, which may bypass the blood-brain barrier and allow exogenous proteins to access the central nervous system directly. Interestingly, it has been reported that intranasal delivery of NGF can ameliorate β-amyloid deposition in animal models of Alzheimer disease and traumatic brain injury [34]. A recent study also confirmed the effectiveness of intranasal administration on promoting neurogenesis in a rat model of cerebral ischemia [35]. Based on these data, in this study we attempted to deliver NGF by the intranasal route in a rat model of ischemic stroke. Our findings show that ischemia-induced angiogenesis in the peri-infarct region is significantly enhanced by the intranasal delivery of NGF.
There are some limitations to our study. We did not observe the long-term effect of NGF on angiogenesis after ischemic stroke. In addition, although we detected the mobilization of EPCs from bone marrow to the peripheral blood, whether NGF can promote EPC migration and homing into the sites of ischemic injury and in turn contribute to neural repair after ischemic injury remains unknown. Further studies are needed to establish in detail the underlying mechanisms of action of NGF on post-stroke angiogenesis.
Conclusion
In the present study, we showed that intranasal administration of NGF promotes angiogenesis in ischemic brain tissue after ischemic stroke. The upregulation of pro-angiogenic factors and mobilization of EPCs from the bone marrow to the peripheral blood may mediate NGF’s angiogenic effect on cerebral ischemia. Our results indicate that NGF-enhanced angiogenesis contributes to neurological functional recovery after ischemic stroke, which may occur through activation of the PI3K/Akt signaling pathway. This study provides novel experimental evidence for the angiogenic roles of NGF in ischemic stroke.
Acknowledgements
This study was supported by the grants from the Natural Science Foundation of Guangdong Province of China (2014A030313681) and the Science and Technology Project of Shenzhen (JCYJ20170307144228252).
Disclosure of conflict of interest
None.
References
- 1.Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- 2.Xia CF, Yin H, Borlongan CV, Chao J, Chao L. Postischemic infusion of adrenomedullin protects against ischemic stroke by inhibiting apoptosis and promoting angiogenesis. Exp Neurol. 2006;197:521–530. doi: 10.1016/j.expneurol.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 3.Bai Q, Lyu Z, Yang X, Pan Z, Lou J, Dong T. Epigallocatechin-3-gallate promotes angiogenesis via up-regulation of Nfr2 signaling pathway in a mouse model of ischemic stroke. Behav Brain Res. 2017;321:79–86. doi: 10.1016/j.bbr.2016.12.037. [DOI] [PubMed] [Google Scholar]
- 4.Liang Z, Brooks J, Willard M, Liang K, Yoon Y, Kang S, Shim H. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem Biophys Res Commun. 2007;359:716–722. doi: 10.1016/j.bbrc.2007.05.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ling L, Zhang S, Ji Z, Huang H, Yao G, Wang M, He R, Deng W, Fang L. Therapeutic effects of lipo-prostaglandin E1 on angiogenesis and neurogenesis after ischemic stroke in rats. Int J Neurosci. 2016;126:469–477. doi: 10.3109/00207454.2015.1031226. [DOI] [PubMed] [Google Scholar]
- 6.Seo K, Choi J, Park M, Rhee C. Angiogenesis effects of nerve growth factor (NGF) on rat corneas. J Vet Sci. 2001;2:125–130. [PubMed] [Google Scholar]
- 7.Emanueli C, Salis MB, Pinna A, Graiani G, Manni L, Madeddu P. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation. 2002;106:2257–2262. doi: 10.1161/01.cir.0000033971.56802.c5. [DOI] [PubMed] [Google Scholar]
- 8.Turrini P, Gaetano C, Antonelli A, Capogrossi MC, Aloe L. Nerve growth factor induces angiogenic activity in a mouse model of hindlimb ischemia. Neurosci Lett. 2002;323:109–112. doi: 10.1016/s0304-3940(02)00090-3. [DOI] [PubMed] [Google Scholar]
- 9.Diao YP, Cui FK, Yan S, Chen ZG, Lian LS, Guo LL, Li YJ. Nerve growth factor promotes angiogenesis and skeletal muscle fiber remodeling in a murine model of hindlimb ischemia. Chin Med J. 2016;129:313–9. doi: 10.4103/0366-6999.174496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, He C, Zhou T, Huang Z, Zhou L, Liu X. NGF increases VEGF expression and promotes cell proliferation via ERK1/2 and AKT signaling in Müller cells. Mol Vis. 2016;22:254–63. [PMC free article] [PubMed] [Google Scholar]
- 11.Steinle JJ, Granger HJ. Nerve growth factor regulates human choroidal, but not retinal, endothelial cell migration and proliferation. Auton Neurosci. 2003;108:57–62. doi: 10.1016/S1566-0702(03)00151-6. [DOI] [PubMed] [Google Scholar]
- 12.Jadhao CS, Bhatwadekar AD, Jiang Y, Boulton ME, Steinle JJ, Grant MB. Nerve growth factor promotes endothelial progenitor cell-mediated angiogenic responses. Invest Ophthalmol Vis Sci. 2012;53:2030–2037. doi: 10.1167/iovs.11-8430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meloni M, Caporali A, Graiani G, Lagrasta C, Katare R, Van Linthout S, Spillmann F, Campesi I, Madeddu P, Quaini F. Nerve growth factor promotes cardiac repair following myocardial infarction. Circ Res. 2010;106:1275–1284. doi: 10.1161/CIRCRESAHA.109.210088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 15.Ling L, Hou Q, Xing S, Yu J, Pei Z, Zeng J. Exogenous kallikrein enhances neurogenesis and angiogenesis in the subventricular zone and the peri-infarction region and improves neurological function after focal cortical infarction in hypertensive rats. Brain Res. 2008;1206:89–97. doi: 10.1016/j.brainres.2008.01.099. [DOI] [PubMed] [Google Scholar]
- 16.Tian L, Guo R, Yue X, Lv Q, Ye X, Wang Z, Chen Z, Wu B, Xu G, Liu X. Intranasal administration of nerve growth factor ameliorate β-amyloid deposition after traumatic brain injury in rats. Brain Res. 2012;1440:47–55. doi: 10.1016/j.brainres.2011.12.059. [DOI] [PubMed] [Google Scholar]
- 17.Cheng S, Ma M, Ma Y, Wang Z, Xu G, Liu X. Combination therapy with intranasal NGF and electroacupuncture enhanced cell proliferation and survival in rats after stroke. Neurol Res. 2009;31:753–758. doi: 10.1179/174313209X382557. [DOI] [PubMed] [Google Scholar]
- 18.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38:3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23:166–180. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- 21.Krupinski J, Issa R, Bujny T, Slevin M, Kumar P, Kumar S, Kaluza J. A putative role for platelet-derived growth factor in angiogenesis and neuroprotection after ischemic stroke in humans. Stroke. 1997;28:564–573. doi: 10.1161/01.str.28.3.564. [DOI] [PubMed] [Google Scholar]
- 22.Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, Risau W. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156:965–976. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1α plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 25.Perrucci GL, Straino S, Corlianò M, Scopece A, Napolitano M, Berk BC, Lombardi F, Pompilio G, Capogrossi MC, Nigro P. Cyclophilin a modulates bone marrow-derived CD117+ cells and enhances ischemia-induced angiogenesis via the SDF-1/CXCR4 axis. Int J Cardiol. 2016;212:324–335. doi: 10.1016/j.ijcard.2016.03.082. [DOI] [PubMed] [Google Scholar]
- 26.Xie Y, Qi Y, Zhang Y, Chen J, Wu T, Gu Y. Regulation of angiogenic factors by the PI3K/Akt pathway in A549 lung cancer cells under hypoxic conditions. Oncol Lett. 2017;13:2909–2914. doi: 10.3892/ol.2017.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Du D, Wang H, Liu Y, Lai X, Jiang F, Chen D, Zhang Y, Zong J, Li Y. Silent information regulator 1 (SIRT1) promotes the migration and proliferation of endothelial progenitor cells through the PI3K/Akt/eNOS signaling pathway. Int J Clin Exp Pathol. 2015;8:2274. [PMC free article] [PubMed] [Google Scholar]
- 28.Song ZY, Wang F, Cui SX, Qu XJ. Knockdown of CXCR4 inhibits CXCL12-induced angiogenesis in HUVECs through downregulation of the MAPK/ERK and PI3K/AKT and the Wnt/betacatenin pathways. Cancer Invest. 2018;36:10–18. doi: 10.1080/07357907.2017.1422512. [DOI] [PubMed] [Google Scholar]
- 29.Graiani G, Emanueli C, Desortes E, Van Linthout S, Pinna A, Figueroa CD, Manni L, Madeddu P. Nerve growth factor promotes reparative angiogenesis and inhibits endothelial apoptosis in cutaneous wounds of Type 1 diabetic mice. Diabetologia. 2004;47:1047–1054. doi: 10.1007/s00125-004-1414-7. [DOI] [PubMed] [Google Scholar]
- 30.Marlin MC, Li G. Biogenesis and function of the NGF/TrkA signaling endosome. Int Rev Cell Mol Biol. 2015;314:239–257. doi: 10.1016/bs.ircmb.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kar S, Samii A, Bertalanffy H. PTEN/PI3K/Akt/VEGF signaling and the cross talk to KRIT1, CCM2, and PDCD10 proteins in cerebral cavernous malformations. Neurosurg Rev. 2015;38:229–237. doi: 10.1007/s10143-014-0597-8. [DOI] [PubMed] [Google Scholar]
- 32.Ahluwalia A, Jones MK, Brzozowski T, Tarnawski AS. Nerve growth factor is critical requirement for in vitro angiogenesis in gastric endothelial cells. Am J Physiol Gastrointest Liver Physiol. 2016;311:G981–G987. doi: 10.1152/ajpgi.00334.2016. [DOI] [PubMed] [Google Scholar]
- 33.Nicoli S, Knyphausen CP, Zhu LJ, Lakshmanan A, Lawson ND. miR-221 is required for endothelial tip cell behaviors during vascular development. Dev Cell. 2012;22:418–429. doi: 10.1016/j.devcel.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capsoni S, Malerba F, Carucci NM, Rizzi C, Criscuolo C, Origlia N, Calvello M, Viegi A, Meli G, Cattaneo A. The chemokine CXCL12 mediates the anti-amyloidogenic action of painless human nerve growth factor. Brain. 2016;140:201–217. doi: 10.1093/brain/aww271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu W, Cheng S, Xu G, Ma M, Zhou Z, Liu D, Liu X. Intranasal nerve growth factor enhances striatal neurogenesis in adult rats with focal cerebral ischemia. Drug Deliv. 2011;18:338–343. doi: 10.3109/10717544.2011.557785. [DOI] [PubMed] [Google Scholar]