Abstract
Purpose:
To determine the objective response rate and response duration of melanoma brain metastases to adoptive cell therapy (ACT) with autologous antitumor lymphocytes plus interleukin-2 following a lymphodepleting preparative regimen.
Methods:
Between 2000 and 2009, 264 patients with metastatic melanoma received ACT, consisting of cyclophosphamide and fludarabine with or without total body irradiation, followed by the infusion of autologous tumor-infiltrating lymphocytes (TIL) or autologous peripheral blood lymphocytes retrovirally transduced to express a T-cell receptor (TCR) that recognized the melanocyte differentiation antigens gp-100 or MART-1. From this group, 26 patients were retrospectively identified to have had untreated brain metastases and extracranial disease before receiving ACT. The response rate and duration of melanoma brain metastases, as well as the overall response rate, response duration, and survival for these patients, are presented.
Results:
Seventeen of these 26 patients received ACT with TIL. Seven of these patients (41%) achieved a complete response in the brain, and six patients achieved an overall partial response. In the nine patients that received TCR-transduced lymphocytes, two patients achieved a complete response in the brain (22%) and one of these two achieved an overall partial response. One patient developed a tumor-associated subarachnoid hemorrhage during the thrombocytopenic phase of therapy and had an uneventful metastatectomy.
Conclusion:
ACT with a nonmyeloablative preparative regimen using either TIL- or TCR gene–transduced cells and interleukin-2 can mediate complete and durable regression of melanoma brain metastases. This strategy can be used safely in selected patients with metastatic melanoma to the brain.
Malignant melanoma commonly metastasizes to the brain. It has been estimated that up to 75% of patients with metastatic melanoma ultimately develop brain metastases (1). Because current systemic treatments for brain metastases are limited and largely ineffective, these patients have a poor prognosis. Current strategies include supportive care, surgical resection, stereotactic radiosurgery, whole brain radiation, chemotherapy, or combinations of these agents. Resection (2) and radiosurgery (3) can produce effective palliation in selected cases, but this is usually restricted to patients with few lesions. Radiation therapy is the current standard of care for individuals with multiple brain metastases; it can improve neurologic symptoms but does not alter survival (4). Objective responses of melanoma brain metastases are rarely seen with chemotherapy or immunotherapy (5, 6). Consequently, it is not surprising that brain metastases are the direct cause of death in 60% to 70% of affected patients (7).
Over the last decade, a major focus of the Surgery Branch at the National Cancer Institute (NCI) has been the investigation of adoptive cell therapy (ACT) using tumor infiltrating lymphocytes (TIL) or T-cell receptor (TCR)-transduced lymphocytes and interleukin-2 (IL-2) with a nonmyeloablative conditioning regimen. The intensity of the preparative regimen was modified in some protocols to include 2-, 6-, or 12-Gy total body irradiation (TBI), and autologous stem cell support was added in those cases. We have also used a variety of cell culture techniques to accelerate TIL growth and to enrich CD8+ lymphocyte subpopulations. The objective response rates in nonmyeloablative regimen alone, nonmyeloablative regimen plus 2-Gy TBI, and nonmyeloablative regimen plus 12-Gy TBI were 49%, 52%, and 72%, respectively (8). Tumor regression has been observed at all visceral and soft tissue sites including the brain. Because TIL cannot be generated for all patients, we have also investigated the infusion of peripheral blood lymphocytes that were transduced with retroviral vectors to express a TCR that recognized either gp-100 or MART-1 in HLA-A2+ patients (9). The objective response rates for these trials have been reported to be 20% to 30%, and durable tumor regression at all disease sites including the brain has also been observed (9, 10).
Typically, 4 to 6 weeks of cell culture are required to obtain adequate numbers of reactive lymphocytes for any of our clinical protocols. As a consequence, there may be a 6- to 8-week interval between the brain magnetic resonance imaging evaluation, which was generally obtained by the referring oncologist for screening, and the protocol baseline images obtained immediately before the start of chemotherapy. The majority of patients in this report developed brain metastases during this short interval.
Patients and Methods
Between 2000 and 2009, 264 patients with metastatic melanoma were enrolled in one of a series of sequential trials designed to investigate ACT with TIL or TCR and high-dose IL-2 along with a nonmyeloablative conditioning regimen. All patients had measurable metastatic melanoma and were enrolled on a protocol that was approved by the NCI Investigational Review Board. Eligible patients were 18 years of age or older; had a life expectancy of at least 3 months; had an Eastern Cooperative Oncology Group score of 0 or 1; had an adequate hepatic, renal, and hematopoietic reserve; and had brain lesions ≤10 mm without significant edema, mass effect, or symptoms. From this population, 26 patients were retrospectively identified to have had untreated and evaluable brain metastases as a component of their disease before treatment. For these patients, we elected to proceed with ACT if the brain metastases were asymptomatic (<10 mm) and showed no mass effect or significant edema. Patient age range, sex, and sites of extracranial disease are shown in Tables 1 and 2.
Table 1.
Patients who received a nonmyeloablative (with or without TBI) adoptive cell transfer with TIL
| Patient no. |
Cells | Prep | TBI (cGy) |
Age/ Sex |
Other disease sites | Cells (109) |
IL-2 dose |
Brain | Overall | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Size (mm) |
Res | Dur (mo) |
Res | Dur (mo) |
||||||||
| 1 | TIL | Cy/Flu | None | 55/M | AX | 104.0 | 5 | 5 | 4 | CR | 6 | PR | 4 |
| 2 | TIL | Cy/Flu | None | 28/M | LI, LU | 60.5 | 6 | 1 | 7 | NR | NR | ||
| 3 | TIL | Cy/Flu | None | 50/F | CL, IL, RP, BR, SU, LU | 10.8 | 10 | 1 | 6 | NR | NR | ||
| 4 | TIL | Cy/Flu | None | 17/M | AX, IL, ME, SK, SU, AD, LI, LU, SP | 74.1 | 1 | 4 | 9 | NR | NR | ||
| 5 | NT | Cy/Flu | None | 61/M | AX, LI, LU | 93.1 | 4 | 2 | 3 | CR | 26+ | PR | 14 |
| 6 | NT | Cy/Flu | None | 59/M | IL, ME, SU, LU | 11.1 | 13 | 4 | 4 | CR | 6 | NR | |
| 7 | YT | Cy/Flu | None | 45/M | MS, AX, CE, CL, ME, MT, PE, Kl | 39.9 | 8 | 1 | 3 | NR | NR | ||
| 8 | NT | Cy/Flu | None | 39/M | LI, SP | 38.0 | 9 | 1 | 5 | NR | NR | ||
| 9 | CD8YT | Cy/Flu | None | 40/M | HI, LU | 51.1 | 5 | 2 | 5 | CR | 14+ | PR | 14+ |
| 10 | CD8YT | Cy/Flu | None | 53/F | HI, ME, LI | 69.8 | 5 | 2 | 4 | CR | 5+ | PR | 5+ |
| 11 | CD8YT | Cy/Flu | None | 35/F | AX, MT, RP, SU, LI, SB | 37.4 | 7 | 1 | 8 | NR | NR | ||
| 12 | CD8 YT | Cy/Flu | None | 32/M | BO, ME, PP, RP, AD, LU | 41.4 | 5 | 6 | 8 | NR | NR | ||
| 13 | CD8 YT | Cy/Flu | None | 55/F | BN, IL, IN, PE, SU, LU, PL | 30.8 | 5 | 1 | 9 | NR | PR | 9 | |
| 14 | TIL | Cy/Flu | 200 | 34/F | BO, SU, IP, LU | 73.7 | 6 | 1 | 5 | CR | 43+ | PR | 4 |
| 15 | CD8YT | Cy/Flu | 600 | 25/F | MS, RP, SU, LU, SP | 51.7 | 5 | 2 | 10 | CR | 4+ | PR | 4+ |
| 16 | CD8YT | Cy/Flu | 600 | 32/F | BO, AD | 97.5 | 7 | 2 | 4 | NR | NR | ||
| 17 | CD8YT | Cy/Flu | 600 | 31/F | BO, IN, RP, EA, EY, SU, Kl, LU, PL, PV | 32.5 | 6 | 5 | 4 | NR | NR | ||
Abbreviations: Prep, preparative chemotherapy regimen; Dur, duration of response; mm, size of largest brain lesion in millimeters; AX, axilla; LI, liver; LU, lung; CL, clavicular; IL, iliac; RP, retroperitoneal; BR, breast; SU, subcutaneous; ME, mediastinal; SK, skin; AD, adrenal; SP, spleen; IN, inguinal; CE, cervical; MT, mesentery, PE, periaortic; KI, kidney; HI, hilar; SB, small bowel; BO, bone; PP, periportal; IP, intraperitoneal; MS, muscle; EA, ear; EY, eye; PL, pleura; PV, pelvis; NR, no response; YT, young TIL; Cy, cyclophosphamide; Flu, fludarabine.
Table 2.
Patients who received a nonmyeloablative (with or without TBI) adoptive cell transfer with TCR
| Patient no. |
Cells | Prep | TBI (cGy) |
Age/ Sex |
Other disease sites | Cells (109) |
IL-2 dose |
Brain | Overall | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Size (mm) |
Res | Dur (mo) |
Res | Dur (mo) |
||||||||
| 1 | F4 | Cy/Flu | None | 32/F | SK | 4.7 | 5 | 1 | 5 | NR | NR | ||
| 2 | F5 | Cy/Flu | None | 46/M | AX, LU | 5.9 | 7 | 2 | 7 | CR | 25+ | PR | 25+ |
| 3 | F5 | Cy/Flu | None | 30/M | BO, AX, MT, LI, LU, PA, PV, HI | 112 | 8 | 1 | 5 | CR | 8 | NR | |
| 4 | F5 | Cy/Flu | None | 31/F | BR.SU | 73.7 | 8 | 1 | 2 | NR | NR | ||
| 5 | F5 | Cy/Flu | None | 43/F | IP, CE, CL, IL, IN, PE, SU, LU | 21.5 | 6 | 4 | 2 | NR | NR | ||
| 6 | gp100 | Cy/Flu | None | 25/F | ME, MS, LU, PL | 59.5 | 5 | 1 | 5 | NR | NR | ||
| 7 | gp154 | Cy/Flu | None | 40/F | MS, IL, MT, RP | 2.7 | 10 | 1 | 6 | NR | NR | ||
| 8 | gp154/F5 | Cy/Flu | 600 | 56/F | HI, ME, LU | 24.5 | 4 | 1 | 5 | NR | NR | ||
| 9 | gp154/F5 | Cy/Flu | 600 | 52/M | ME, EA, NK, SU, LU | 63.0 | 6 | 1 | 5 | NR | NR | ||
Abbreviations: Prep, preparative chemotherapy regimen; Dur, duration of response; mm, size of largest brain lesion in millimeters. AX, axilla; LI, liver; LU, lung; CL, clavicular; IL, iliac; RP, retroperitoneal; BR, breast; SU, subcutaneous; ME, mediastinal; SK, skin; AD, adrenal; SP, spleen; IN, inguinal; CE, cervical; MT, mesentery, PE, periaortic; KI, kidney; HI, hilar; SB, small bowel; BO, bone; PP, periportal; IP, intraperitoneal; MS, muscle; EA, ear; EY, eye; PL, pleura; PV, pelvis; PA, pancreas; NK, neck; Cy, cyclophosphamide; Flu, fludarabine.
Conditioning regimen
The conditioning regimens have been previously described in detail (11). In brief, the regimen consisted of cyclophosphamide (60 mg/kg/d on days −7 and −6) and fludarabine (25 mg/m2/d on days −5 to −1). In some protocols, additional lymphodepletion with 2, 6, or 12 Gy was added at 1 to 3 days immediately before cell transfer. Moreover, chemotherapy was compressed, overlapping the two agents. Patients received the lymphocyte infusion on day 0 along with IL-2 (720,000 IU/kg every 8 hours) as tolerated to a maximum of 15 doses. Platelet counts were generally maintained by transfusion at ≥20,000/μL for patients with known brain metastases. Patients were reevaluated at all known tumor sites including the brain approximately 4 to 6 weeks after the initiation of treatment and subsequently at 4- to 8-week intervals.
Cell products used for therapy
Patients received an autologous lymphocyte product manufactured in the Surgery Branch Cell Production Facility that passed a protocol-specific certificate of analysis, including potency, safety, and sterility criteria. Patients listed in Table 1 received TIL cultures derived from resected metastatic melanoma lesions. TIL cultures for infusion were generated as described previously (12). Briefly, resected specimens were enzymatically disaggregated into single-cell suspensions, or small tumor fragments were separated and plated to initiate cultures. TIL were propagated in 6,000 IU/mL IL-2 in complete medium containing 10% human serum until a homogenous lymphocyte culture evolved. Some cultures (patients 1–4 and 13, TIL, Table 1) were highly selected by assessing tumor recognition and other cultures (patients 9–12, 15–17, CD8 YT, Table 1) were grown from the entire cell population and were CD8+ enriched (13) before final expansion. Bulk, tumor-reactive, or CD8+-enriched TIL were rapidly expanded with anti-CD3 antibody, IL-2, and irradiated peripheral blood mononuclear “feeder” cells for 14 days before patient infusion (14, 15). Patients listed in Table 2 received a genetically retargeted product derived from peripheral blood lymphocytes. Peripheral blood lymphocytes from leukocytopheresis were stimulated with anti-CD3 and IL-2 then transduced with a recombinant retrovirus encoding the α and β chains of an HLA-A2 restricted, tumor antigen–reactive TCR genes. Cells were monitored for transduction efficiency and tumor reactivity, then further rapidly expanded with anti-CD3, IL-2, and irradiated feeder cells. TCRs used for these studies include the HLA-A2/MART-1:27–35–specific “F4” and “F5” (9), HLAA2/gp100:209–217–specific “gp100” (16), and HLA-A2/gp100:154–162–specific “g154” (10).
Statistical analysis
Objective response rates (partial and complete), duration of response, and time to progression (time from treatment to disease progression) were calculated, and overall survival probabilities (treatment to last follow-up or death) were estimated using the Kaplan-Meier method. Data were last analyzed in March 1, 2010. Patient response was assessed using standard radiographic studies and physical examination at 4 weeks after cell administration and at regular intervals thereafter. Responses were categorized into complete (CR), partial (PR), or no response based on Response Evaluation Criteria in Solid Tumors guidelines (17). Although the brain metastases were small (≤10 mm), the standard Response Evaluation Criteria in Solid Tumors was also used to evaluate the brain response; however, ultimately, no PR was encountered. The duration of response was determined from the date patients received the cell infusion.
Results
Brain response and overall response to TIL
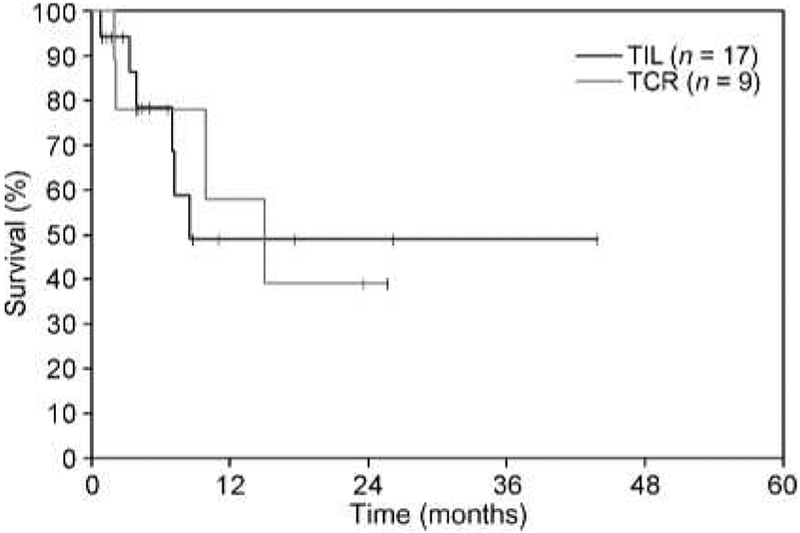
Seventeen patients that received TIL with a lymphodepleting preparative regimen and bolus IL-2 had evaluable brain metastases. Seven (41%) of these patients achieved CR of all brain disease, and five of these responses are continuing in the brain at 4 to 44 months (Table 1; Fig. 1). Patients 1 and 6, who experienced CR in the brain for 6 months, ultimately developed diseases at new sites in the brain as well as at extracranial sites. Five of these seven patients had at least two brain metastases, and two individuals had four or more metastases. All lesions that responded to ACT were 10 mm or less in size. Although all of the patients had extensive extracranial metastatic disease, six of the seven patients with CR in the brain achieved an overall PR. The median survival for these 17 patients was 8.5 months, with an estimated actuarial 2-year survival of approximately 40% (Fig. 3).
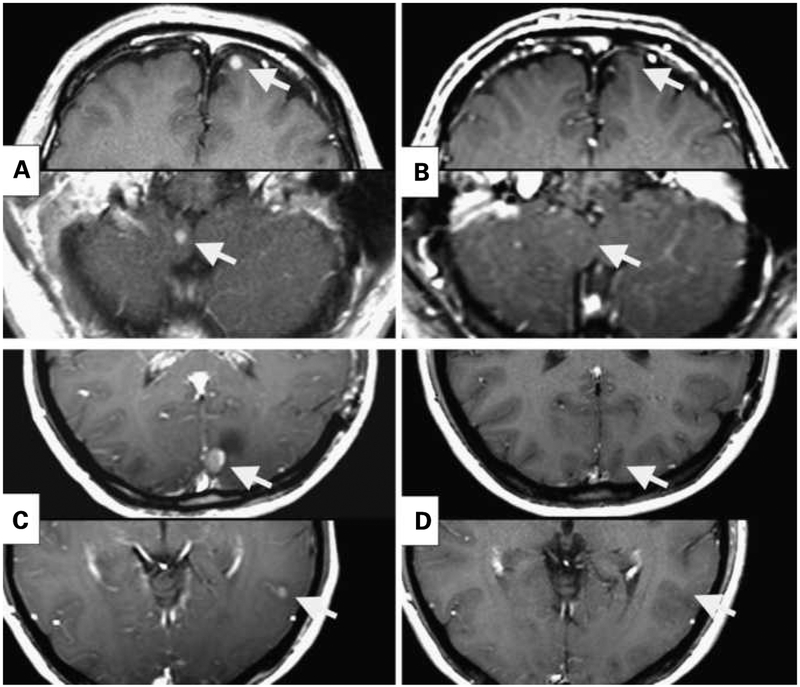
Fig. 1.
Resolution of multiple brain lesions in two patients treated with CD8-enriched TIL and IL-2 after preparative lymphodepleting regimens of Cy/Flu (A and B) or Cy/Flu + 6-Gy TBI (C and D). A and C, pretreatment magnetic resonance imaging. B, 14 months after cell transfer. D, 6 months after cell transfer.
Fig. 3.
Kaplan-Meier estimates of overall survival for melanoma patients with untreated brain metastases before ACT.
One patient in this group (patient 13) developed a subarachnoid hemorrhage of a 9-mm intracranial lesion located in the right insular cortex during the thrombocytopenic phase of treatment. This patient had an elective uncomplicated craniotomy and metastatectomy when the thrombocytopenia resolved, and is considered a nonresponder in the brain. However, this patient had extracranial regression and achieved an overall PR for 9 months and had no neurologic sequelae.
Brain response and overall response to TCR
Nine patients that received TCR gene–transduced lymphocytes with a lymphodepleting preparative regimen and IL-2 had evaluable brain metastases. Two of these patients achieved CR in the brain, one lasting 8 months and the other continuing at 25 months (Table 2; Fig. 2). Patient 3, who experienced CR in the brain for 8 months, ultimately developed new sites of brain disease as well as developed diseases at extracranial sites. One of these patients had two brain lesions, and the other had one brain lesion. One of the two patients with CR in the brain developed an overall objective PR, whereas the other patient had a brief minor regression extracranially. None of these patients developed significant neurotoxicity as a result of the preparative regimen or lymphocyte infusion with IL-2. The median survival for these nine patients was 15 months (Fig. 3).
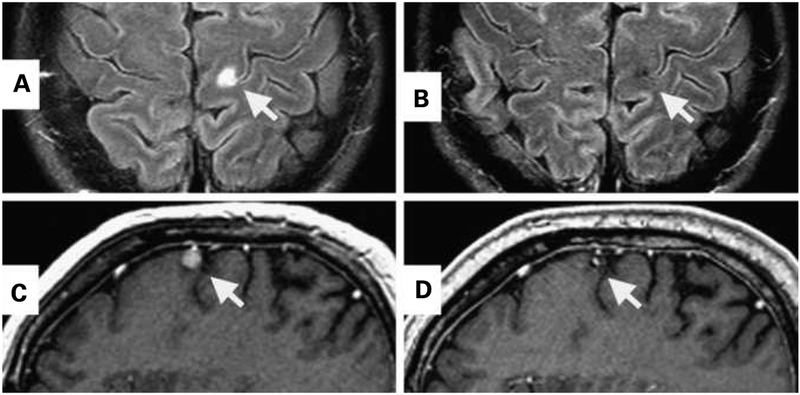
Fig. 2.
CR of a brain metastasis in two patients treated with anti–MART-1 TCR-transduced peripheral blood lymphocytes and IL-2 following a preparative regimen of Cy/Flu. A and C, pretreatment magnetic resonance imaging. B, 6 months after cell transfer. D, 30 months after cell transfer.
Discussion
The treatment of brain metastases from melanoma remains a difficult clinical challenge. The prognosis for patients with brain metastases is poor, with the median survival ranging from 2.8 to 4.0 months after diagnosis(18). The management of these patients is complicated by the need to treat both central nervous system (CNS) metastases as well as other systemic diseases.
Resection or stereotactic radiosurgery of solitary melanoma brain metastases either with or without whole-brain radiation therapy (WBRT) is recognized as the treatment of choice. Retrospective surgical series suggest that the median survival for patients with resected solitary lesions is approximately 5 to 8 months (2, 19, 20). Retrospective series using WBRT after surgical resection in patients with melanoma brain metastases report no survival benefit (2, 21,22). The results of stereotactic radiosurgery seem to be comparable to resection, with a median survival for patients having solitary and multiple brain metastases ranging from 5 to 9 months (3). WBRT in concert with radiosurgery seems to prevent new lesions and neurologic causes of death, but has no impact on survival largely due to uncontrolled extracranial disease (23). Conversely, chemotherapy alone has been largely unsuccessful in treating melanoma brain metastases. A multicenter phase II study of temozolomide in 151 patients with brain metastases from melanoma reported a 7% response rate in the brain with a median survival of 3.5 months (5). Similarly, immunotherapy with high-dose bolus IL-2 has also been ineffective. One retrospective study at the NCI reported an overall response rate of 5.6% for patients with previously untreated brain metastases. Two of 36 patients with evaluable brain metastases had objective regression of intracranial and extracranial disease after receiving IL-2 (6). Ipilimumab has recently been reported to cause the CR of untreated melanoma brain metastases in two patients (24, 25). A phase II trial that investigated the treatment of melanoma brain metastases with ipilimumab reported that of 51 patients with melanoma brain metastases, 5 developed PR in the brain (26).
We have previously reported that ACT with autologous antitumor lymphocytes plus bolus IL-2 following a lymphodepleting preparatory regimen can cause objective regression of metastatic melanoma in 49% to 72% of patients (8). The retrospective analysis of patients with both untreated brain metastasis and uncontrolled extra‐ cranial disease confirms that this therapy can mediate complete and durable regression of metastatic melanoma in the brain. Seven of 17 patients that received TIL and 2 of 9 patients that received TCR achieved CR in the brain. Of the 26 patients, 15 were alive at the time of analysis. Five of these 15 patients survived beyond 12 months without additional CNS or systemic therapy. One patient with four brain lesions achieved CR that has been durable for more than 18 months. The median survival of patients that received TCR and TIL in this analysis was noted to be 15 and 8.5 months, respectively. A group of similar patients without untreated melanoma brain metastases that received TIL showed a median survival of 16.6 months (8).
Our patients were carefully selected, and we treated only patients with brain lesions ≤10 mm that had no evidence of significant edema, mass effect, or symptoms before cell therapy. The largest lesion that responded to this therapy was 10 mm. The response rate of larger lesions to this treatment approach is unknown but needs to be investigated. Interestingly, all of the intracranial tumor regressions were complete. In addition, seven of the nine patients that had brain response also achieved an overall objective response. Conversely, all patients that failed to achieve a response in the brain also failed to achieve an overall objective response at other visceral sites. This suggests that metastases in the brain are as responsive to ACT as those in extracranial sites. Moreover, it confirms that activated lymphocytes can effectively traffic to the CNS.
ACT is typically associated with a brief period of thrombocytopenia, and platelet transfusions are required. One patient (patient 13) developed a subarachnoid hemorrhage during the thrombocytopenic phase of therapy. The platelet count was 26,000/μL when the patient became symptomatic with a severe headache. Workup revealed hemorrhage and no evidence of aneurysm. This patient had an uncomplicated craniotomy 10 days later when the thrombocytopenia resolved. We suspected that this bleed was caused by tumor necrosis from therapy due to the presence of extensive lymphocytic infiltration in the tumor specimen. Subsequent to this event, we have attempted to maintain a platelet count ≥30,000/μL for patients with untreated brain metastases. No other patient experienced neurologic complication from rapid CNS disease progression during TIL or TCR therapy. Patients who did not achieve brain response after ACT ultimately required resection, stereotactic radiosurgery (SRS), or WBRT depending on the extent of their CNS progression. Interestingly, although nine patients developed CR in the brain, it should be noted that none of these patients developed an overall CR to this treatment.
ACT with autologous antitumor lymphocytes plus high-dose IL-2 following a lymphodepleting preparative regimen can mediate complete and durable regression of metastatic melanoma in the brain and can result in long-term survival. Furthermore, this therapy is safe in selected patients, and the presence of melanoma brain metastases is not necessarily a contraindication to ACT therapy. These results show that ACT is unique in its ability to achieve high objective response rates at both intracranial and extracranial sites, and many of these responses seem to be durable.
Translational Relevance.
Metastatic melanoma to the brain is a difficult and frequent clinical challenge. Current systemic treatments for patients with melanoma and brain involvement are limited and largely ineffective. Adoptive cell therapy (ACT) with a nonmyeloablative preparative regimen using activated lymphocytes and interleukin-2 is an experimental approach that has been shown to be effective for some patients with metastatic melanoma. This report describes our experience in using ACT for patients with metastatic melanoma to both the brain and extra-cranial sites. We document that ACT can mediate complete and durable regression of untreated brain metastasis, which can also result in long-term survival for some patients. These results suggest that activated lymphocytes can effectively traffic to the central nervous system. This strategy is important because it affords the opportunity to simultaneously address metastatic melanoma to both the brain and extracranial sites.
Acknowledgments
We thank the members of the Surgery Branch immunotherapy team of nurses, data managers, and other fellows who contributed to this effort.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Harrison BE, Johnson JL, Clough RW, Halperin EC. Selection of patients with melanoma brain metastases for aggressive treatment. Am J Clin Oncol 2003;26:354–7. [DOI] [PubMed] [Google Scholar]
- 2.Sampson JH, Carter JH, Jr., Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg 1998;88:11–20. [DOI] [PubMed] [Google Scholar]
- 3.Gaudy-Marqueste C, Regis JM, Muracciole X, et al. Gamma-knife radiosurgery in the management of melanoma patients with brain metastases: a series of 106 patients without whole-brain radiotherapy. Int J Radiat Oncol Biol Phys 2006;65:809–16. [DOI] [PubMed] [Google Scholar]
- 4.Seung SK, Sneed PK, McDermott MW, et al. Gamma knife radiosurgery for malignant melanoma brain metastases. Cancer J Sci Am 1998;4:103–9. [PubMed] [Google Scholar]
- 5.Agarwala SS, Kirkwood JM, Gore M, et al. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol 2004;22:2101–7. [DOI] [PubMed] [Google Scholar]
- 6.Guirguis LM, Yang JC, White DE, et al. Safety and efficacy of high-dose interleukin-2 therapy in patients with brain metastases. J Immunother 2002;25:82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg 1978;135:807–10. [DOI] [PubMed] [Google Scholar]
- 8.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regiments. J Clin Oncol 2008;26: 5233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006;314:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 2009;114:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following nonmyeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol 2005;23:2346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother 2003;26:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prieto PA, Durflinger KH, Wunderlich JR, Rosenberg SA, Dudley ME. Enrichment of CD8+ cells from melanoma tumor infiltrating lymphocyte cultures reveals tumor reactivity for use in adoptive cell therapy. J Immunother 2010;33:547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 2002;298:850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods 1990;128:189. [DOI] [PubMed] [Google Scholar]
- 16.Burns WR, Zheng Z, Rosenberg SA, Morgan RA. Lack of specific γ-retroviral vector long terminal repeat promoter silencing in patients receiving genetically engineered lymphocytes and activation upon lymphocyte restimulation. Blood 2009;114:2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. , European Organization for Research and Treatment of CancerNational Cancer Institute for the United StatesNational Cancer Institute of Canada. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2000;92:205–16. [DOI] [PubMed] [Google Scholar]
- 18.Byrne TN, Cascino TL, Posner JB. Brain metastasis from melanoma. J Neurooncol 1983;1:313–7. [DOI] [PubMed] [Google Scholar]
- 19.Zacest AC, Besser M, Stevens G, Thompson JF, McCarthy WH, Culjak G. Surgical management of cerebral metastases from melanoma: outcome in 147 patients treated at a single institution over two decades. J Neurosurg 2002;93:552–8. [DOI] [PubMed] [Google Scholar]
- 20.Wronski M, Arbit E. Surgical treatment of brain metastases from melanoma: a retrospective study of 91 patients. J Neurosurg 2000;93:9–18. [DOI] [PubMed] [Google Scholar]
- 21.Hagen NA, Cirrincione C, Thaler HT, DeAngelis LM. The role of radiation therapy following resection of single brain metastasis from melanoma. Neurology 1990;40:158–60. [DOI] [PubMed] [Google Scholar]
- 22.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 1998;280:1485–9. [DOI] [PubMed] [Google Scholar]
- 23.Ellerhorst J, Strom E, Nardone E, McCutcheon I. Whole brain irradiation for patients with metastatic melanoma: a review of 87 cases. Int J Radiat Oncol Biol Phys 2001;39:93–7. [DOI] [PubMed] [Google Scholar]
- 24.Schartz NE, Farges C, Madelaine I, et al. Complete regression of a previously untreated melanoma brain metastasis with ipilimumab. Melanoma Res 2010;20:247–50. [DOI] [PubMed] [Google Scholar]
- 25.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockage in patients with metastatic melanoma. PNAS 2003;100: 8372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawrence DP, Hamid O, McDermott DF, et al. Phase II trial of ipilimumab monotherapy in melanoma patients with brain metastases. J Clin Oncol 2010;28:15.19933920 [Google Scholar]