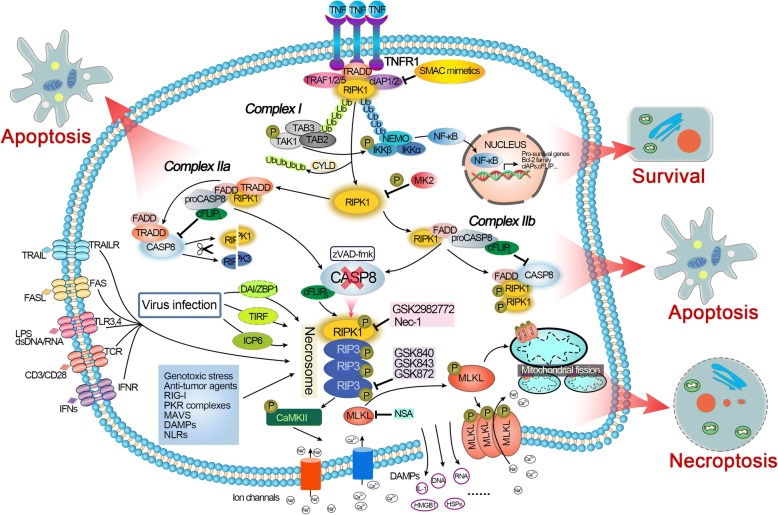
Fig. 2.
A schematic overview of the molecular signaling pathways involved in necroptosis. Upon TNF-α stimulation, activated TNFR1 recruits various downstream proteins, including RIPK1, to form prosurvival complex I, resulting in RIPK1 polyubiquitination and subsequently facilitating NF-κB signaling to prevent cell death (see text). Phosphorylation of RIPK1 by MK2 can also limit RIPK1 activation and the subsequent assembly of the death complex through the IKKα/β independent way. Inhibition of cIAPs (by Smac or Smac mimetics) leads to CYLD-mediated deubiquitination of RIPK1 and its dissociation from TNFR1, resulting in the formation of different prodeath complexes (complex IIa, IIb and the necrosome). Complex IIa contains TRADD and can be formed independently of the scaffold and kinase function of RIPK1. In contrast, complex IIb lacks TRADD and requires RIPK1 kinase activity for cell death induction. Complex IIa and IIb activate caspase-8, leading to apoptotic cell death. If caspase-8 activity is blocked, RIPK1 will bind to RIPK3 to form necrosomes and promote RIPK3 autophosphorylation and activation. Activated RIPK3 is currently known to function via at least two downstream effectors: MLKL and CaMKII, which are effector molecules leading to necroptosis through multiple mechanisms. Other stimuli, including FasL, TRAIL, CD3/CD28, LPS, dsDNA/RNA and IFNs, can stimulate their corresponding receptors to activate necrosomes to promote necroptosis. Infection with some viruses directly activates RIPK3 through DAI, TIRF or ICP6. Anticancer agents, genotoxic stress and some other factors can also trigger RIPK1/RIPK3-dependent necroptosis. Necroptosis is inhibited experimentally by specific inhibitors of RIPK1, RIPK3 and MLKL, as shown above