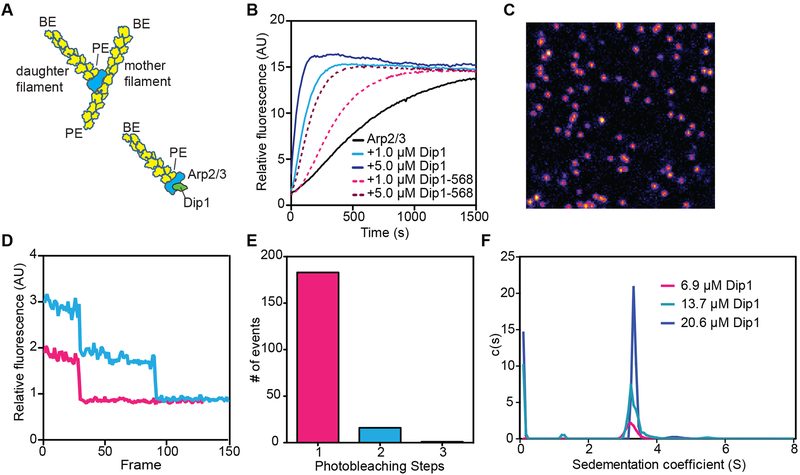
Figure 1: Model of Dip1-mediated Arp2/3 complex activation and characterization of Alexa Fluor 568 labeled Dip1.
A. Cartoon of a branched filament nucleated by WASP-activated Arp2/3 complex (top) showing resulting filament polarities (barbed end, BE; pointed end, PE). Bottom half of panel shows a model of Dip1-mediated activation of Arp2/3 complex. In this model, the linear filament nucleated by Dip1-bound Arp2/3 complex is analogous to the daughter filament nucleated during branching nucleation. B. Time course of polymerization of 3 μM 15% pyrene-labeled actin in the presence of 50 nM S. pombe Arp2/3 complex (SpArp2/3 complex) and either unlabeled or Alexa Fluor 568 labeled Dip1 (568-Dip1). C. Representative image of 568-Dip1 molecules bound to a coverslip and visualized by TIRF microscopy. D. Examples of fluorescence intensities of single 568-Dip1 puncta over time. E. Quantification of events in which 568-Dip1 photobleached in one versus multiple steps. F. Plot of c(S) versus (S) for sedimentation velocity analytical ultracentrifugation of three concentrations of unlabeled Dip1. Dip1 sediments predominantly or entirely as a monomer at all three concentrations. See also Table S1.