Summary
Vertebrate ocular microbiomes are poorly characterized and virtually unexplored in wildlife species. Pathogen defense is considered a key function of microbiomes, but determining microbiome stability during disease is critical for understanding the role of resident microbial communities in infectious disease dynamics. Here, we characterize the ocular bacterial microbiome of house finches (Haemorhous mexicanus), prior to and during experimental infection with an inflammatory ocular disease, Mycoplasmal conjunctivitis, caused by Mycoplasma gallisepticum. In ocular tissues of healthy house finches, we identified 526 total bacterial operational taxonomic units (OTUs, 97% similarity), primarily from Firmicutes (92.6%) and Proteobacteria (6.9%), via 16S rRNA gene amplicon sequencing. Resident ocular communities of healthy female finches were characterized by greater evenness and phylogenetic diversity compared to healthy male finches. Regardless of sex, ocular microbiome community structure significantly shifted 11 days after experimental inoculation with M. gallisepticum. A suite of OTUs, including taxa from the genera Methylobacterium, Acinetobacter, and Mycoplasma, appear to drive these changes, indicating that the whole finch ocular microbiome responds to infection. Further study is needed to quantify changes in absolute abundance of resident taxa and to elucidate potential functional roles of the resident ocular microbiome in mediating individual responses to this common songbird bacterial pathogen.
Introduction
Animals host diverse symbiotic microbes that are now recognized as crucial for many organismal functions, including ATP and nutrient biosynthesis (The Human Microbiome Consortium, 2012), immune system maturation and responsiveness (MacPherson et al., 2000; Mazmanian et al., 2005), and intestinal epithelial cell turnover (Rakoff-Nahoum et al., 2004). Much is known about the vertebrate gut microbiome because of the abundance of microbes in the gastrointestinal tract and their essential role in digestion. Comparatively, the structure and function of vertebrate ocular microbiomes are poorly characterized. The eye, conjunctiva, lens, and tears in healthy humans appear to harbor diverse bacteria (Graham et al., 2007; Dong et al., 2011; Hutchinson et al., 2014; Shin et al. 2016). There is some debate about whether these ocular microbes represent transient colonizers or a stable, active community (Zegans and Van Gelder, 2014), but the consistency of bacterial taxa found across studies is strongly suggestive of a stable human ocular microbiome (Kugadas and Gadjeva, 2016; Shin et al., 2016). Both culture-based and next-generation sequencing studies have found that Gram-positive bacterial genera in the phyla Proteobacteria and Actinobacteria, including Pseudomonas, Propionibacterium, and Corynebacterium, tend to dominate human ocular communities (Dong et al., 2011; Smith et al., 2013; Wilcox, 2013; Zhou et al., 2014). Furthermore, resident ocular microbiomes in humans have been shown to differ by sex: male ocular microbiomes tend to be dominated by Actinobacteria and Firmicutes, while female ocular microbiomes are dominated by Proteobacteria (Shin et al., 2016). To date, the resident ocular microbiomes of vertebrate wildlife remain virtually unexplored (but see Santos et al., 2014; Alfano et al., 2015). However, the low diversity of these microbiomes make them particularly well suited for characterizing individual-level variation and elucidating potential function.
A key function of mucosal microbiomes is host pathogen defense (McDermott et al., 2014), and determining whether microbiomes are stable or in flux during disease has been identified as a critical benchmark for understanding the function of ocular microbiomes (Zegans and Van Gelder, 2014). The eye, itself, is often considered to be immune privileged (i.e., antigens are tolerated and inflammatory and local immune responses are limited to prevent tissue damage; Zhou and Caspi, 2010). Interactions between commensal microbiota and the ocular environment may therefore be critical in maintaining homeostasis of the eye (Miller and Lovieno, 2009). Furthermore, it has been suggested that disruption of the microbiome of the cornea and conjunctiva may open a pathway for ocular disease (Abelson et al., 2015). Across diverse types of tissues, including the nose, gut, and skin, reductions in microbiome diversity have been associated with the development of and severity of infection (Chang et al. 2008, Biswas et al., 2015; Holden et al., 2015; Sekirov et al., 2008). There are also numerous examples of resident microbiomes responding to pathogens (e.g. reduced gut biodiversity in swine following viral diarrheal infection [Koh et al., 2015]; reduced microbial diversity in reef-building corals infected with white plague disease [Cardenas et al., 2012]), and the ocular microbiomes of Sprague-Dawley rats were found to change in response to a non-infectious disease, type I diabetes mellitus [Yang et al., 2015]. Overall, revealing the extent to which the composition of the microbiota shifts during infection is critical to understanding the role of the microbiome in disease outcome.
Here, we characterize the ocular microbiome of a common North American songbird species, house finches (Haemorhous mexicanus), prior to and during experimental infection with a debilitating inflammatory ocular pathogen, Mycoplasma gallisepticum (MG), which causes Mycoplasmal conjunctivitis. A distinct clade of the poultry bacterial pathogen MG has caused annual epidemics in eastern North American house finches since the mid-1990s (Dhondt et al., 1998) and causes conjunctivitis in house finches (Luttrell et al., 1998). The house finch-MG system is therefore particularly well suited for exploring how disease may influence the ocular microbiome in a wild vertebrate. Our study had two overarching goals: first, to characterize the resident ocular microbiome in healthy house finches, and to test whether resident ocular communities vary with sex, as was recently detected in human ocular microbiomes (Shin et al. 2016). Second, we aimed to test how experimental inoculation with low doses of MG alters the resident ocular microbiome of finches.
Results
Characterizing healthy ocular bacterial communities of house finches
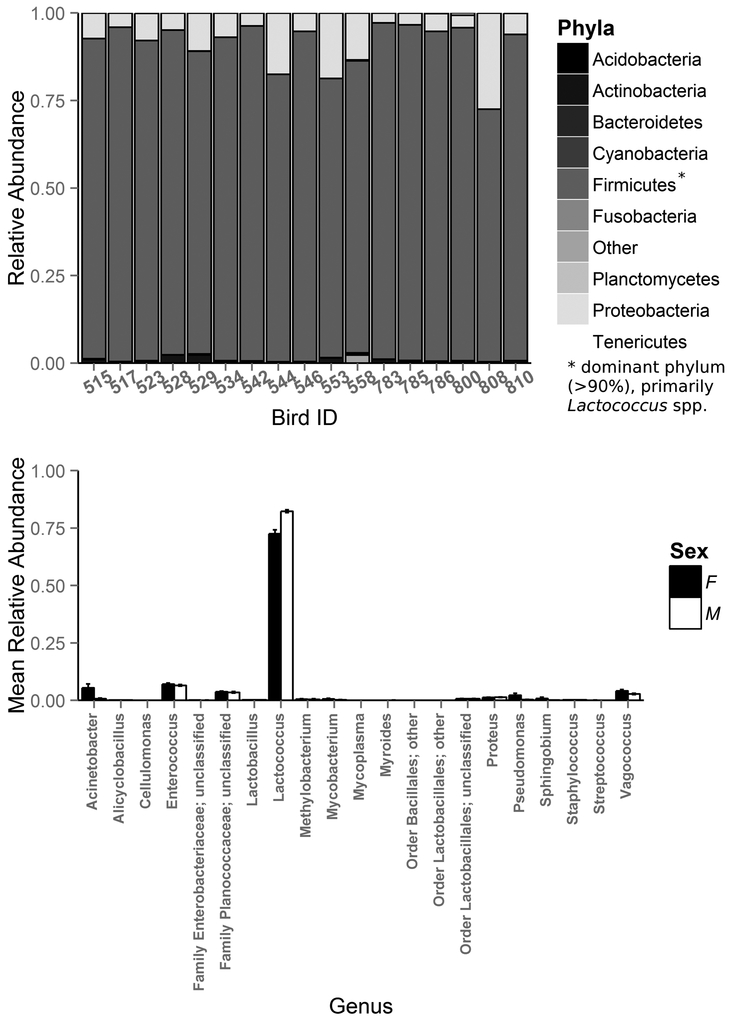
Using 16S Illumina sequencing, we identified 526 total bacterial OTUs in the ocular samples of all healthy house finches (n=17) included in this study. Predominant identified phyla included Firmicutes (mean relative abundance ± SD = 92.6% ± 6.65), Proteobacteria (6.9% ± 6.67), Actinobacteria (0.5% ± 0.57), Bacteroidetes (0.1% ± 0.06), and Tenericutes (<0.1% ± 0.02; Fig. 1a), which comprised 20 different genera present in all samples (Fig. 1b). The resident ocular microbiomes of healthy house finches were dominated by bacteria from the genera Lactococcus (77.1% ± 6.42), Enterococcus (6.8% ± 1.26), Vagococcus (3.5% ± 1.46), and Acinetobacter (3.2% ± 4.49). In fact, these 4 genera comprised over 90% of the relative abundance of the resident ocular community.
Figure 1.
Mean relative abundances of (a) bacterial phyla present in the complete healthy microbiome of wild house finches (Haemorhous mexicanus) and (b) bacterial genera present in all ocular samples of healthy male (open bars) and female (closed bars) H. mexicanus.
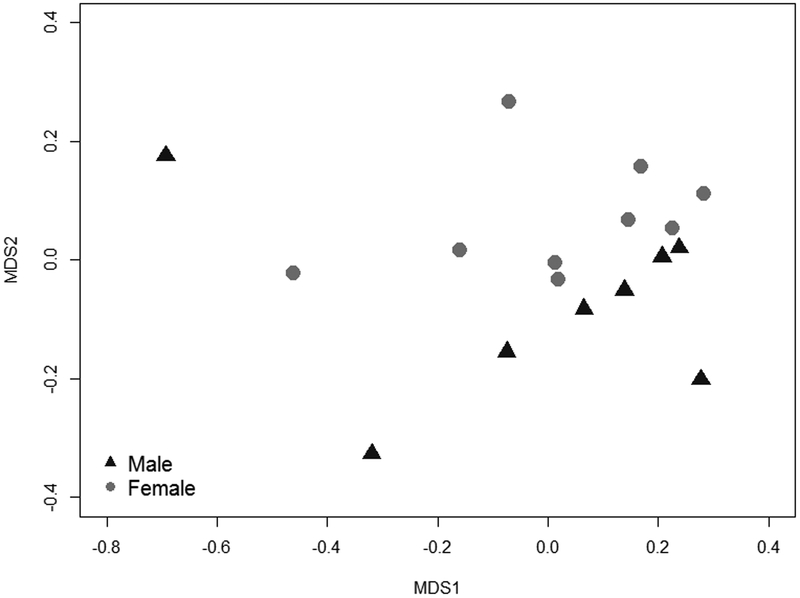
Ocular bacterial communities differed between healthy male (n = 8) and female (n = 9) finches; female finches harbored a greater phylogenetic diversity of bacteria compared to males (Faith’s phylogenetic diversity, t = 2.40, df = 14.95, p = 0.03; mean phylogenetic distance ± SD: female: 12.7 ± 1.1 and male: 11.4 ± 1.0). Female ocular communities were also significantly more even (Simpson index, t = 5.09, df = 12.28, p = 0.0002; mean ± SD: female: 0.30 ± 0.03, male: 0.23 ± 0.01) than male ocular communities (Fig. 1b). These differences appear to be driven by a higher relative abundance of Lactococcus spp. in the ocular microbiomes of males than females (79.7% relative abundance in males versus 69.6% in females; t = −5.28, df = 10.13, p = 0.0003). Based on PERMANOVA and NMDS ordination, OTU community structure also differed significantly between healthy males and females (NMDS stress: 0.1022, adonis pseudo F = 6.57, R2 = 0.30, p < 0.0001; Fig. 2).
Figure 2.
Community structure of healthy house finch (Haemorhous mexicanus) ocular bacterial communities varied significantly by sex. Stress = 0.10
Influence of M. gallisepticum infection on ocular bacterial communities
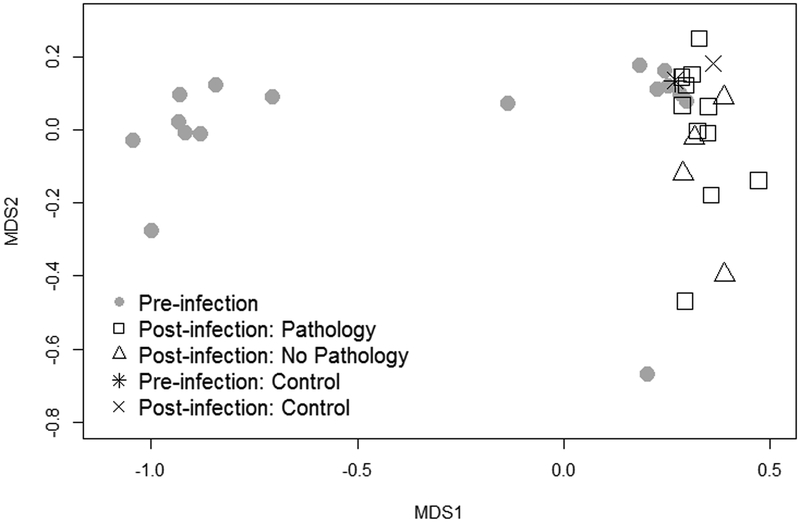
Following characterization of the resident ocular microbiome, house finches were inoculated with one of two low doses of MG: 3.0 × 102 CCU/mL (males n = 4; females n = 4) or 3.0 × 103 CCU/mL (males n = 4; females n = 4) of MG. Ocular bacterial community structure on day 11 post-inoculation significantly differed from that of healthy birds (NMDS stress: 0.0675; Date: adonis pseudo F = 16.85, R2 = 0.35, p < 0.0001), with no effect of sex either alone or in interaction with time (Sex: adonis pseudo F = 0.17, R2 = 0.005, p = 0.710; Date × Sex: adonis pseudo F = 0.37, R2 = 0.01, p = 0.527; Fig. 3). There was also no effect of inoculation dose on community structure (Dose × Date: adonis pseudo F = 0.11, R2 = 0.01, p = 0.896) despite significantly higher absolute levels of MG (measured via quantitative PCR) on day 11 in birds given the higher of two infective doses (mean log MG: 3.0 × 102 CCU/mL = 2.82 ± 0.78, 3.0 × 103 CCU/mL = 5.11 ± 0.43; Dose: R2 = 0.30, F1, 13 = 7.05, p = 0.020).
Figure 3.
Beta diversity of house finch (Haemorhous mexicanus) ocular microbial communities before and after experimental inoculation with Mycoplasma gallisepticum. Pre-inoculation communities are represented by grey circles and post-inoculation communities by open symbols. Though four birds did not develop pathology (open triangles), MG was detected at low levels in these individuals on day 11 post-inoculation (see Fig 4). The single control bird (ID #529) is included for reference and is represented by a * (pre-infection) and × (post-infection). Stress = 0.07
The detected differences in community structure following MG inoculation are likely largely driven by changes in the relative abundance of MG, which became a dominant community member in most birds post-infection. Indeed, absolute abundance of MG in our conjunctival swabs as detected by our MG-specific qPCR was also a strong predictor of Illumina read number of Mycoplasma OTUs, a measure of relative abundance based on rarefaction of the dataset (R2 = 0.97, F1, 14 = 528.9, p < 0.0001; Fig. 4). However, we used the K-S measure, a variable screening method extension of Kolmogorov-Smirnov statistics, to identify OTUs driving community-level differences and found that Mycoplasma was not solely responsible for the detected differences in pre- versus post-inoculation bacterial communities. Nine OTUs, including Mycoplasma (OTU X694430), comprised the final set of OTUs defining differences between pre- and post-infection communities, with K-S measures ranging from 0.34 to 0.50 (Table 1). Interestingly, five OTUs had larger K-S measures than Mycoplasma (OTU X694430), suggesting that bacteria other than Mycoplasma contributed strongly to the changes in post-infection community structure (Table 1, Fig. 5). The OTU with the highest K-S measure (X4396717), indicating that it contributed most strongly to differences between pre- and post-inoculation ocular communities, was a Methylobacterium species. Most of the remaining OTUs identified as drivers of post-inoculation community composition were Acinetobacter species (X103411, X1790396, denovo3638, and denovo2394). OTUs from the Families Methylobacteriaceae, Mycobacteriaceae, Moraxellaceae, and Sphingomonadaceae experienced significant shifts in relative abundance after MG inoculation, as the confidence intervals for the mean changes in relative abundance do not cross zero (Fig 5b).
Figure 4.
Absolute abundance of Mycoplasma gallisepticum (MG) in house finch conjunctival swabs on day 11 post-inoculation, as quantified by qPCR, significantly predicts the number of Illumina 16S rRNA amplicon ‘reads’ from the same day 11 samples (R2 = 0.97). Though four birds did not develop pathology (open triangles), MG was detected in all birds (black circles) on day 11 post-inoculation.
Table 1.
K-S measures and taxonomic information for nine OTUs that best define differences in the pre- and post-infection ocular microbiomes of house finches (Haemorhous mexicanus) before and after infection with Mycoplasma gallisepticum.
| OTU | K-S measure |
Phylum | Class | Order | Family | Genus |
|---|---|---|---|---|---|---|
| X4396717* | 0.50 | Proteobacteria | Alphaproteobacteria | Rhizobiales | Methylobacteriaceae | Methylobacterium |
| X550712* | 0.50 | Actinobacteria | Actinobacteria | Actinomycetales | Mycobacteriaceae | Mycobacterium |
| X103411* | 0.47 | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | Acinetobacter |
| X1790396* | 0.44 | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | Acinetobacter |
| X4393057* | 0.44 | Proteobacteria | Alphaproteobacteria | Sphingomonadales | Sphingomonadaceae | Sphingobium |
| X694430 | 0.43 | Tenericutes | Mollicutes | Mycoplasmatales | Mycoplasmataceae | Mycoplasma |
| denovo3638* | 0.37 | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | Acinetobacter |
| denovo2394* | 0.37 | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | Acinetobacter |
| denovo2964 | 0.34 | Tenericutes | Mollicutes | Mycoplasmatales | Mycoplasmataceae | Unclassified |
indicates OTUs with K-S measures that remain in the top nine taxa that define differences between pre- and post-infection ocular microbiomes when Mycoplasma gallisepticum (bolded) is removed from the analysis.
Figure 5.
(a) A heatmap illustrating relative abundance of nine OTUs shown to be driving differences between the ocular microbiome pre- and post-experimental infection with Mycoplasma gallisepticum. The intensity of color indicates the relative abundance of these OTUs prior to experimental inoculation (left) in the “healthy” resident microbiome and following experimental inoculation (right). Birds are ordered from lowest to highest pathology (left to right) at day 11 post-inoculation. Although the single control individual (ID: 529) is included in this figure as a visual baseline, this individual was excluded from statistical analysis of K-S scores driving differences pre- and post-infection. Though four birds did not develop pathology following inoculation (highlighted in black boxes), the ocular community still changed. Relative abundance is shown on a log scale. (b) Mean change in relative abundance of the eight non-MG OTUs responsible for pre- and post-infection differences in the ocular microbiome of all 16 inoculated house finches. Note that the scales on the x axes differ. * denotes significant changes (standard error does not cross zero) in relative abundance. (c) Mean change in relative abundance of the same eight non-MG OTUs in the four birds that did not develop pathology after inoculation with MG. Circles represent changes in these OTUs in the control bird (ID: 529) for comparison. Note that the scales on the x axes differ. * denotes significant differences (>2 standard deviations different) between the control bird and the birds that did not develop pathology.
To further confirm that changes in relative abundance of other taxa were not solely driven by an increasing relative abundance of MG, we recalculated relative abundances after removing MG from the community. Using this subtracted community, K-S scores and OTU identity were consistent for 7 of 9 OTUs that defined the differences between pre- and post-infection microbiomes (Table S1). Thus, the detected changes in the ocular microbiome post-inoculation appear to be driven by a suite of ocular community members, including but not limited to Mycoplasma.
Discussion
Here we characterized the ocular bacterial microbiome of captive house finches before and after experimental inoculation with a common bacterial ocular pathogen, MG. We found that the resident ocular microbiomes of house finches was comprised of 526 total bacterial OTUs representing only 9 phyla, and only 4 genera comprised 90% of the total relative abundance. For comparison, human ocular microbiomes have been found to be comprised of 6 phyla dominated by upwards of 10 genera (Dong et al., 2011; Kugadas and Gadjeva, 2016; Shin et al., 2016), while koala ocular microbiomes were dominated by 5 phyla and 3–4 genera representing over 90% of the relative abundance (Alfano et al., 2015), rat ocular microbiomes were comprised of 8 genera that represent 100% of ocular bacterial diversity (Yang et al., 2015), and bat ocular microbiomes were comprised of 8 culturable taxa representing 100% of bacterial diversity (Santos et al, 2014). House finch ocular bacterial community diversity was comparable to other non-human vertebrate animals, while human ocular bacterial communities were characterized by a greater diversity of genera by comparison.
The resident house finch ocular bacterial communities were dominated primarily by Lactococcus spp. in the Phylum Firmicutes. In contrast, the few vertebrate ocular microbiomes that have been characterized (humans, lab rats, and koalas) were found to be dominated by members of the Phyla Actinobacteria and Proteobacteria (Dong et al., 2011; Alfano et al., 2015; Yang et al. 2015; Kugadas and Gadjeva, 2016; Shin et al., 2016). Although Firmicutes have been identified in these microbiomes as well, they are at much lower relative abundance in comparison to the house finch ocular microbiome (Dong et al., 2011; Lee et al., 2012; Zhou et al., 2014). Lactococcus species, members of the group of lactic acid producing bacteria (LAB) and the most dominant genus in the house finch ocular microbiome, are known to be beneficial members of the microbiomes of humans and other animals, including honeybees (Vasquez et al., 2012), because they inhibit the growth of pathogenic bacteria and fungi via production of antimicrobial metabolites and host immune modulation (Ventura et al., 2009). Lactococcus spp. may play a similar protective role in the eyes of house finches, but further work is needed in order to characterize the potential function of Lactococcus spp. in house finch conjunctiva.
We found sex differences in the resident ocular microbiomes of healthy house finches, where female finch microbiomes were characterized by higher phylogenetic diversity and evenness than male microbiomes. These differences in evenness appear to be largely driven by relative abundance of the dominant community member, Lactococcus spp, which was higher in males. Sex differences were recently documented in human ocular microbiomes (Shin et al. 2016) and have been documented for a suite of microbiomes in other tissues including the skin and gut (Fierer et al 2008, Markle et al. 2013). In some cases, these difference may contribute to known sex biases in the development of autoimmune and inflammatory disease (Whitacre et al., 1999; Zandman et al., 2007; Markle et al., 2013; Yurkovetskiy et al., 2013). A prior field study detected sex differences in the prevalence of Mycoplasmal conjunctivitis: hatch year female house finches had higher rates of conjunctivitis than hatch year males (Altizer et al. 2004). However, an experimental study using a high inoculation dose of MG did not find any sex differences in the severity of conjunctivitis (Kollias et al. 2004). Overall, further work is needed to confirm the detected sex differences in the resident ocular microbiome and to determine whether these differences influence the likelihood or severity of MG in house finches.
House finch ocular communities were found to differ significantly after experimental inoculation with MG. These pre- and post-inoculation community differences are likely largely driven by the addition of the bacterium MG; in fact, the absolute abundance of MG in house finch conjunctiva was a strong predictor of the relative abundance measured by amplicon sequencing (Fig. 4). However, the changes in ocular communities during MG infection do not appear to be driven by Mycoplasma alone; eight other OTUs were found to drive these community-level differences pre- vs. post-inoculation. In fact, when communities were compared after MG was removed from the dataset and relative abundances were recalculated, these pre-and post-infection differences persisted (Table S1), suggesting that post-inoculation community structure may be driven by a suite of microbes. Together, these results suggest that direct or indirect interactions between MG and commensal bacteria may be occurring during infection. However, genera-specific qPCR assays are needed to definitely determine how commensal bacteria change in absolute abundance during MG infection. Intriguingly, a few individuals did not develop pathology in response to MG inoculation despite pathogen loads indicating that these birds might have become infected at low levels (see Fig. 4), but there was still evidence of change in the relative abundance of eight non-MG OTUs in these individuals (see Fig. 5). However, further study with a larger control group is needed to determine if the post-inoculation changes in the birds that did not develop pathology are truly in response to MG inoculation and not representative of natural temporal fluctuations.
We used a statistical metric, the K-S measure, to identify OTUs that were responsible for observed differences in the communities of birds prior to and following experimental infection. The OTUs with the highest K-S measure, even when Mycoplasma remained in the dataset, were Methylobacterium and Acinetobacter species. Methylobacterium spp. (Methylobacteriaceae_X4396717) have been identified as members of the core microbiome of human eyes (Dong et al., 2011; Shin et al., 2016). However, they have also been reported as opportunistic pathogens that can form biofilms (Kovaleva et al., 2014). Acinetobacter spp. (Moraxellaceae_X103411) also form biofilms, but they are regularly found on human skin as well. Some have been correlated with lower allergy incidence (DeBarry et al., 2010; Hanski et al., 2012) and documented as core microbiota of human eyes (Dong et al., 2011). Because MG is also known to form biofilms (Chen et al., 2012), it may be that its addition to the ocular community resulted in the depletion of resources at the conjunctival surface necessary for biofilm formation, resulting in the observed decreases in relative abundance of other bacteria that form biofilms. However, in addition to direct interference, microbial community changes can result from blocking access to attachment sites (Collado et al., 2008; Rendueles et al., 2012), interbacterial warfare via production of antibiotics, bacteriocins, or other metabolites (Ohland and Macnaughton, 2010; Short et al., 2014), or immune mediated-modulation (Higgins and Johnson, 2001). Chemical signals produced during interbacterial interactions can result in subtle manipulation of the competitor to the benefit of the signal producer; many of these interactions, including chemical-based interactions, often rely on the close proximity of bacterial cells that is likely during biofilm formation (Short et al., 2014). Overall, further study is needed to confirm changes in absolute abundance of resident ocular taxa following MG inoculation and to investigate the potential mechanisms underlying these changes.
Overall, our results suggest that the resident ocular microbiome of house finches differs by sex and shifts in response to MG inoculation. Future work should determine whether the ocular microbiome communities of captive finches, as characterized here, reflect those of free-living birds. Effects of captivity on gut and skin microbiomes have been detected in past studies (e.g., Becker et al., 2014; Kohl et al., 2014; Bataille et al., 2016). However, wild and captive amphibian skin communities retain 70% similarity even after multiple generations in captivity (Becker et al. 2014), suggesting that ocular communities for free-living and captive finches are likely to be largely similar even if effects of captivity are present. Future work should also experimentally determine whether the resident ocular microbiome of house finches is important for regulating host responses to MG infection. Our results suggest that the observed post-inoculation community changes are not solely a result of domination of the microbiome by MG, and further study should investigate potential direct and indirect interactions between MG and commensal bacteria during infection.
Experimental Procedures
Host Species Capture and Housing
Sixty-six house finches were captured in June-August 2014 in Montgomery County, VA and Williamsburg, VA using cage traps and mist nets (under federal and state permits USFWS MB158404–1 and VDGIF 50352) for a separate study on the effects of repeated low-dose MG exposure on host disease response and immune protection (Leon and Hawley, unpublished). The microbiomes of only a subset (n=17) of the 66 birds in that larger study were characterized here (see Study Design). Birds (n=10) that were captured in Williamsburg, VA were temporarily housed in flocks in outdoor aviaries for a maximum of 3 days and transported to Virginia Tech by state vehicle. All finches were then pair-housed at constant day length and temperature, and were fed ad libitum pelleted diet (Daily Maintenance Diet, Roudybush Inc., Woodland, CA). Eighteen days prior to inoculation, all finches were moved to individual cages for the duration of the experiment. All birds were blood-sampled and screened via ELISA, and only those found to be seronegative for MG at capture (following Hawley et al., 2011) were included in experiments. All animal capture, housing, and sampling procedures were approved by the Institutional Animal Care and Use Committee of Virginia Tech (BIOL-12–124).
Study Design
In order to characterize the “healthy” ocular microbiome of house finches, we used 17 birds (8 males, 9 females) that were sampled 14 days prior to experimental inoculation with MG. To examine how the ocular microbiome changes with MG infection, those same 17 birds were then resampled on day 11 post-inoculation (the date of peak pathology; Fig S1), after receiving one of the following 3 treatments: inoculation with 3.0 ×102 CCU/mL of MG (n=8; 4 males, 4 females), inoculation with 3.0×103 CCU/mL dose of MG (n=8; 4 males, 4 females), or sham inoculation of bacteria media (Frey’s broth media with 15% swine serum) alone (n=1; female).
Experimental Inoculation
House finches were inoculated in the palpebral conjunctiva of both eyes with a total volume of 70 µL of either MG diluted in Frey’s broth media with 15% swine serum (FMS) at a total concentration of approximately 3.0 ×102 or 3.0 ×103 CCU/mL or FMS alone (sham inoculation). The MG isolate used, VA1994, was isolated from a house finch captured in Virginia in 1994 (7994–1 7P 2/12/09; D.H. Ley, North Carolina State University, College of Veterinary Medicine, Raleigh, NC, USA, Ley et al., 1996). Dilutions were calculated from the starting viable count of 2.24×107 CCU/mL. Inoculum was maintained at −80°C and was not diluted until immediately after thawing.
Conjunctival Sampling
To characterize both pre- and post-inoculation ocular bacterial communities, as well as to assess MG pathogen load via qPCR, each bird’s conjunctiva was swabbed by the same individual for 5s with a sterile cotton swab dipped in sterile tryptose phosphate broth (TPB). Immediately after swabbing, swabs were swirled in 300μL of sterile TPB and then wrung out into the tube to collect as much conjunctival fluid as possible for subsequent DNA extraction. Samples from both eyes were pooled in one 300µl aliquot of TPB within sample date for a given individual and were then frozen at −20°C until further processing.
Molecular methods
Genomic DNA was extracted from conjunctival samples (TPB broth) with Qiagen DNeasy 96 Blood and Tissue kits (Qiagen, Valencia, CA). The extracted genomic DNA from each sample was used as the template for two assays: a qPCR assay to measure overall numbers of MG present in the conjunctival sample, and 16S rRNA gene amplicon sequencing to characterize the bacterial community of the house finch eye. MG pathogen load was assessed using a qPCR assay targeting the mgc2 gene of MG using the primers from (Grodio et al., 2008) and qPCR methods outlined in (Adelman et al., 2013). 16S rRNA gene amplicon sequencing of the V4 region was done using primers 515F and barcoded 806R (Caporaso et al., 2012).
PCR reactions for amplicon sequencing consisted of 12 μL molecular grade PCR water, 10 μL 5 Prime Hot Master Mix, 0.5 μL each of the forward and reverse primers (10 μM final concentration), and 2.0 μL genomic DNA. PCR conditions included a denaturation step of 3 min at 94 °C, an amplification step for 35 cycles for 45 s at 94 °C, annealing for 60 s at 50 °C, extension for 90 s at 72 °C and a final extension of 10 min at 72 °C. For each sample, we also ran a control with no template. Each sample was amplified in triplicate reactions, with the triplicates pooled. Each resulting pooled sample was then visualized on a 1.5% agarose gel, quantified with a Qubit 2.0 fluorometer (Invitrogen, Carlsbad, California) and combined into a final single pooled sample containing an equal amount of DNA from each sample for amplicon sequencing. The final pool was cleaned using the Qiagen QIAquick PCR clean up kit (Qiagen, Valencia, CA) and sent for sequencing at the Dana Farber Cancer Institute’s Molecular Biology Core Facility on an Illumina MiSeq using protocols as per Caporaso et al. (2012). Due to anticipated low diversity within these samples, the samples were split among two different sequencing runs and each set was run with samples from another system (amphibian skin/lizard cloacal swabs) and also with a 10% PhiX addition. We used a 250 bp paired-end sequencing strategy, but due to poor quality of reverse reads, we used only the forward reads to produce the final dataset. Because samples were extracted using our standard protocols for quantifying absolute abundance of MG via qPCR, we did not use the bead-beating step during DNA extraction that is now standardly used for microbiome studies (Wu et al., 2010; Shin et al., 2016). Thus, some spore-forming bacterial taxa may be underrepresented in the bacterial communities (Guo and Zhang, 2013).
Resulting amplicon data were processed using the QIIME pipeline (Caporaso et al., 2010). Initial quality filtering allowed no barcode errors and required a phred score of 20. The resulting fasta file was imported into Geneious (version 8.1.8; Biomatters, Ltd.) where 250 bp sequences were selected, and PhiX and house finch mitochondrial sequences were removed using rapid ‘map to reference’. The resulting file of 250 bp sequences was then exported. In QIIME, operational taxonomic unit (OTU) identification was assigned based on 97% similarity of sequences using the UCLUST method (Edgar, 2010), and the most abundant sequence from each cluster was chosen to represent each OTU. Sequences were aligned to the Greengenes 13_8 reference database (DeSantis et al., 2006), and taxonomy was assigned using the RDP classifier (Wang et al., 2007). After the OTU table was generated, any remaining taxa assigned to chloroplast or mitochondria were removed, resulting in a file of 33 samples with a total of 2104714 reads. We then filtered out any OTUs with fewer than 0.001% of the total reads (Bokulich et al., 2012) and rarefied samples at a depth of 30400 reads to standardize sequencing. Rarefaction curves generated from this dataset indicated that rarefaction at 30400 reads was sufficient to represent bacterial community richness. The final dataset consisted of 33 samples (one post-inoculation sample [Band ID: 534] did not amplify; pre-inoculation n=17, post-inoculation n=16), with a total of 526 OTUs, and a range of 81–309/OTUs per bird; this dataset is available in the National Center for Biotechnology Information Sequence Read Archive repository (accession # SRP073372, http://www.ncbi.nlm.nih.gov/sra/SRP073372).
Statistical Analyses
To characterize the healthy ocular bacterial community of house finches, we used Student’s t-tests to assess differences in alpha-diversity, based on OTU richness and phylogenetic diversity, and we used the Simpson index to evaluate differences in ocular community structure between male and female house finches sampled prior to MG inoculation. We also tested whether alpha diversity metrics differed by population origin (Williamsburg vs. Montgomery County), but because no differences were found (p > 0.17 for all metrics), population was not included in any further analyses.
We determined whether bacterial communities changed in response to MG inoculation using multi-dimensional analysis of beta diversity. We limited this analysis to the 16 birds that were exposed to MG (e.g., the single sham control was excluded). First, we asked whether the ocular communities of birds differed prior to and following inoculation (n=32 total samples from 16 birds, each sampled prior to and following infection). Bacterial OTU community data were transformed into distance matrices using Bray-Curtis dissimilarities, and significance was tested using permutational multivariate analysis of variance (PERMANOVA) and the adonis function in package {vegan} (Oksanen et al., 2016). Because no significant differences were found among post-inoculation bacterial communities based on MG inoculation dose (3.0 ×102 or 3.0 ×103 CCU/mL), we pooled individuals across inoculation dose for all subsequent analyses. Results were visualized using non-metric multidimensional scaling (NMDS).
To identify OTUs important for driving differences between pre-inoculation and post-inoculation bacterial communities, we used a novel variable screening method called the K-S measure, an extension of the K-S statistic, as described in Belden et al. (2015) and Loftus et al. (2015). The K-S measure uses weighted sums of the K-S statistics calculated for all pairwise comparisons of the distributions defined by K groups, and ranges in value from 0–1; higher values imply larger differences between K distributions. K-S measures for the ocular OTU community data were calculated and plotted in descending order, and a natural break in K-S measure values detected after the 9th value was used as a cutoff to identify the OTUs most responsible for differences in bacterial communities resulting from MG infection. To ensure that MG, which became a dominant community member for most birds in the study, was not solely driving our results, we repeated this analysis after removing MG from the community and calculating relative abundance of all remaining community members. Finally, we used general linear regression to test whether absolute abundance of Mycoplasma gallisepticum detected by qPCR predicted the Illumina read abundance of Mycoplasma spp. from the same conjunctival swab on day 11 post-inoculation.
Supplementary Material
Originality-Significance Statement.
The house finch-Mycoplasmal conjunctivitis system is a strong wildlife model system for understanding the diversity and function of an understudied microbial community, the ocular microbiome. Mycoplasmal conjunctivitis, caused by the bacterial pathogen Mycoplasma gallisepticum, causes inflammatory ocular disease in wild house finches. While many aspects of Mycoplasmal conjunctivitis pathology and virulence have been characterized in house finches, the ocular microbiome has not previously been studied. Furthermore, information regarding the diversity and composition of vertebrate ocular microbiomes in general, especially in wildlife, is lacking. Here, we characterize the ocular microbiome of house finches at the site of infection, the conjunctival sac, both prior to and during experimentally-induced Mycoplasmal conjunctivitis. Intriguingly, resident ocular bacterial communities in healthy house finches differed significantly between male and female birds, but these differences did not persist after experimental inoculation with M. gallisepticum. Additionally, we found that ocular community composition shifted significantly after pathogen inoculation, and these differences could not be entirely attributed to the addition of the bacterial pathogen itself. These results suggest that pathogen dominance of the ocular microbiome is likely not solely responsible for all infection-related changes in bacterial composition documented in this system, and thus interactions may be occurring between symbiotic bacterial taxa and M. gallisepticum. This work is broadly applicable to researchers interested in microbial community-host-pathogen interactions. Further, because wild animal systems are often better proxies for understanding human and domesticated animal disease than are highly inbred laboratory animal systems, these data are likely to be of interest to scientists in the fields of disease ecology, agriculture, and medicine.
Acknowledgments
This work was funded by NIH grant 5R01GM105245 (to DMH) as part of the joint NIH-NSF-USDA Ecology and Evolution of Infectious Diseases program and NSF grant #1136640 to LKB. AL was supported by funding from DMH’s NIH grant and funding from Virginia Tech’s NIH/NIGMS funded IMSD Program 5R25GM072767–07. We thank Daniel Medina for help preparing samples for Illumina sequencing, and Myra Hughey and Daniel Medina for statistical assistance. We also thank Jim Adelman, Sahnzi Moyers, Courtney Youngbar, Catherine Beach, Johanel Caceres, and David Vasquez for experimental and sample collection assistance for this study. The authors declare that they have no conflicts of interest to report.
References
- Abelson MB, Lane K, and Slocum C (2015). The secrets of ocular microbiomes. In: Review of Ophthalmology Online. [WWW document]. URL http://www.reviewofophthalmology.com/content/t/ocular_disease/c/55178/. [Google Scholar]
- Adelman JS, Carter AW, Hopkins WA, and Hawley DM (2013). Deposition of pathogenic Mycoplasma gallisepticum onto bird feeders: host pathology is more important than temperature-driven increases in food intake. Biol Letters. doi: 10.1098/rsbl.2013.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano N, Courtiol A, Vielgrader H, Timms P, Roca AL, and Greenwood AD (2015). Variation in koala microbiomes within and between individuals: effect of body region and captivity status. Sci Rep. doi:10.1038/srep10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altizer S, Davis AK, Cook KC, and Cherry JJ (2004). Age, sex, and season affect the risk of mycoplasmal conjunctivitis in a southeastern house finch population. Canadian Journal of Zoology. 82: 755–763. [Google Scholar]
- Bataille A, Lee-Cruz L, Tripathi B, Kim H, and Waldman B (2016). Microbiome variation across amphibian skin regions: implications for Chytridiomycosis mitigation efforts. Microb. Ecol. 71: 221. doi:10.1007/s00248-015-0653-0. [DOI] [PubMed] [Google Scholar]
- Belden LK, Hughey MC, Rebollar EA, Umile TP, Loftus SC, Burzynski EA, et al. (2015). Panamanian frog species host unique skin bacterial communities. Front Microbiol. doi:10.3389/fmicb.2015.01171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MH, Richards-Zawacki CL, Gratwicke B, and Belden LK (2014). The effect of captivity on the cutaneous bacterial community of the critically endangered Panamanian golden frog (Atelopus zeteki). Biol. Conserv. 176: 199–206. [Google Scholar]
- Biswas K, Hoggard M, Jain R, Taylor MW, and Douglas RG (2015). The nasal microbiota in health and disease: variation within and between subjects. Front Microbiol. doi:10.3389/fmicb.2015.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, et al. (2012). Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods. doi: 10.1038/nmeth.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat Methods. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. (2012). Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6: 1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas A, Rodriguez-R LM, Pizarro V, Cadavid LF, and Arevalo-Ferro C (2012). Shifts in bacterial communities of two reef-building coral species affected by white plague disease. ISME. 6: 505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JY, Antonopoulos DA, Kalra A, Tonelli A, Khalife WT, Schmidt TM, et al. (2008). Decreased diversity of the fecal microbiome in recurrent Clostridium difficile—associated diarrhea. J Infect Dis. 197: 435–438. [DOI] [PubMed] [Google Scholar]
- Chen H, Yu S, Hu M, Han X, Chen D, Qiu X, and Ding C (2012). Identification of biofilm formation by Mycoplasma gallisepticum. Vet Microbiol. 161: 96–103. [DOI] [PubMed] [Google Scholar]
- Collado MC, Isolauri E, and Salminen S (2008). Specific probiotic strains and their combinations counteract adhesion of Enterobacter sakazakii to intestinal mucus. FEMS Microbiol Letters. 282: 58–64. [DOI] [PubMed] [Google Scholar]
- DeBarry J, Hanuszkiewicz A, Stein K, Holst O, and Heine H (2010). The allergy-protective properties of Acinetobacter lwoffi F78 are imparted by its lipopolysaccharide. Allergy. 65: 690–697. [DOI] [PubMed] [Google Scholar]
- DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. (2006). Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 72: 5069–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhondt AA, Tessaglia DL, and Slothower RL (1998). Epidemic mycoplasmal conjunctivitis in house finches from eastern North America. J Wildl Dis. 34: 265–280. [DOI] [PubMed] [Google Scholar]
- Dong Q, Brulc J, Iovieno A, Bates B, Garoutte A, Miller D, et al. (2011). Diversity of bacteria at healthy human conjunctiva. Invest Ophthalmol Vis Sci. 52: 5408–5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos LL, Montiani-Ferreira F, Lima L, Lange R, and de barros Filho IR (2014). Bacterial microbiota of the ocular surface of captive and free-ranging microbats: Desmodus rotundus, Diameus youngi and Artibeus lituratus. Vet. Opthalmol. 17: 157–161. [DOI] [PubMed] [Google Scholar]
- Edgar RC (2010). Search and clustering orders of magnitude faster than BLAST. Bioinform. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Fierer N, Hamady M, Lauber CL, and Knight R (2008). The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci USA. 105: 17994–17999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J, Moore J, Jiru X, Moore JE, Goodall EA, Dooley JSG, et al. (2007). Ocular pathogen or commensal: a PCR-based study of surface bacterial flora in normal and dry eyes. Invest Ophthalmol Vis Sci. 48: 5616–5623. [DOI] [PubMed] [Google Scholar]
- Grodio JL, Dhondt KV, O’Connell PH, and Schat KA (2008). Detection and quantification of Mycoplasma gallisepticum genome load in conjunctival samples of experimentally infected house finches (Carpodacus mexicanus) using real-time polymerase chain reaction. Avian Pathol. 37: 385–391. [DOI] [PubMed] [Google Scholar]
- Guo F and Zhang T (2013). Biases during DNA extraction of activated sludge samples revealed by high throughput sequencing. Appl Microbiol and Biotechnol. 97: 4607. doi: 10.1007/s00253-012-4244-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski I, von Hertzen L, Fyhrquist N, Koskinen K, Torppa K, Laatikainen T, et al. (2012). Environmental biodiversity, human microiota, and allergy are interrelated. Proc Natl Acad Sci USA. 109: 8334–8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley DM, Grodio J, Frasca S, Kirkpatrick L, and Ley DH (2011). Experimental infection of domestic canaries (Serinus canaria domestica) with Mycoplasma gallisepticum: a new model system for a wildlife disease. Avian Pathol. 40: 321–327. [DOI] [PubMed] [Google Scholar]
- Higgins P and Johnson L (2001). Prior Helicobacter pylori infection ameliorates Salmonella Typhimurium-induced colitis: mucosal crosstalk between stomach and distal intestine. Inflamm. Bowel Dis. 17: 1398–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden WM, Hanlon SM, Woodhams DC, Chappell TM, Wells HL, Glisson SM et al. (2015). Skin bacteria provide early protection for newly metamorphosed southern leopard frogs (Rana sphenocephala) against the frog-killing fungus, Batrachochytrium dendrobatidis. Biol Conserv. 187: 91–102. [Google Scholar]
- Hutchinson DS, Pflugfelder SC, and Petrosino JF (2014). Microbiome, Eye. In: Encyclopedia of Metagenomics. Nelson KE (ed). New York: Springer-Verlag; p. 400–403. [Google Scholar]
- Koh H, Kim MS, Lee J, Kim H, and Park S (2015). Changes in the swine gut microbiota in response to porcine epidemic diarrhea infection. Microbes Environ. 30: 284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl KD, Skopec MM, and Dearing MD 2014. Captivity results in disparate loss of gut microbial diversity in closely related hosts. Conserv Physiol. 2: cou009. doi: 10.1093/conphys/cou009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollias GV, Sydenstricker KV, Kollias HW, Ley DH, Hosseini PR, Connolly V, et al. (2004). Experimental infection of house finches with Mycoplasma gallisepticum. J. Wild. Dis. 40: 79–86. [DOI] [PubMed] [Google Scholar]
- Kovaleva J, Degener JE, and van der Mei HC (2014). Methylobacterium and its role in health care-associated infection. J Clin Microbiol. 52: 1317–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugadas A and Gadjeva M (2016). Impact of microbiome on ocular health. The Ocular Surface. 14: 342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Oh DH, Jung JY, Kim JC, and Jeon CO (2012). Comparative ocular microbial communities in humans with and without blepharitis. Invest Ophthalmol Vis Sci. 53: 5585–5593. [DOI] [PubMed] [Google Scholar]
- Ley DH, Berkhoff JE, and McLaren JM (1996). Mycoplasma gallisepticum isolated from house finches (Carpodacus mexicanus) with conjunctivitis. Avian Dis. 40: 480–483. [PubMed] [Google Scholar]
- Loftus SC, House LL, Hughey MC, Walke JB, Becker MH, and Belden LK (2015). Dimension reduction for Multinomial Models via a Kolmogorov-Smirnov Measure (KSM). [WWW document]. URL http://www.stat.vt.edu/research/Technical_Reports/TechReport15-1.pdf. [Google Scholar]
- Luttrell MP, Stallknecht DE, Fischer JR, Sewell CT, and Kleven SH (1998). Natural Mycoplasma gallisepticum infection in a captive flock of house finches. J Wildl Dis. 34: 289–296. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, and Zinkernagel RM (2000). A Primitive T Cell-Independent Mechanism of Intestinal Mucosal IgA Responses to Commensal Bacteria. Science. 288: 2222–2226. [DOI] [PubMed] [Google Scholar]
- Markle JGM, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, et al. (2013). Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 339: 1084–1088. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, and Kasper DL (2005). An Immunomodulatory Molecule of Symbiotic Bacteria Directs Maturation of the Host Immune System. Cell. 122: 107–118. [DOI] [PubMed] [Google Scholar]
- McDermott AJ and Huffnagle GB (2014). The microbiome and regulation of mucosal immunity. Immunol. 142: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D and Lovieno A (2009). The role of microbial flora on the ocular surface. Curr Opin Allergy Clin Immunol. 9: 466–70. [DOI] [PubMed] [Google Scholar]
- Ohland CL and Macnaughton WK (2010). Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 298: G807–G819. [DOI] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, et al. (2016). Package ‘vegan.’ [WWW document]. URL https://cran.r-project.org/web/packages/vegan/vegan.pdf. [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, and Medzhitov R (2004). Recognition of Commensal Microflora by Toll-Like Receptors Is Required for Intestinal Homeostasis. Cell. 118: 229–241. [DOI] [PubMed] [Google Scholar]
- Rendueles O, Ferrieres L, Fretaud M, Begaud E, Herbomel P, Levraud J, et al. (2012). A New Zebrafish Model of Oro-Intestinal Pathogen Colonization Reveals a Key Role for Adhesion in Protection by Probiotic Bacteria. PLoS Path. doi: 10.1371/journal.ppat.1002815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirov I, Tam NM, Jogova M, Robertson ML, Li Y, Lupp C et al. (2008). Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infection and Immunity. 76: 4726–4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Price K, Albert L, Dodick J, Park L, and Dominguez-Bello MG (2016). Changes in the Eye Microbiota Associated with Contact Lens Wearing. mBio. 7: e00198–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short FL, Murdoch SL, and Ryan RP (2014). Polybacterial human disease: the ills of social networking. Trends in Microbiol. 22: 508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Kennedy S, Gibbs R, Jones D, De Paiva C, Pflugfelder S, et al. (2013). Challenges of microbiome research on the ocular surface. Invest Ophthalmol Vis Sci. 54: 3380. [Google Scholar]
- The Human Microbiome Project Consortium. (2012). Structure, function and diversity of the healthy human microbiome. Nature. 486: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez A, Forsgren E, Fries I, Paxton RJ, Flaberg E, Szekely L, and Olofsson TC (2012). Symbionts as Major Modulators of Insect Health: Lactic Acid Bacteria and Honeybees. PLoS One. doi: 10.1371/journal.pone.0033188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura M, O’Flaherty S, Claesson MJ, Turromi F, Klaenhammer TR, van Sinderen D, et al. (2009). Genome-scale analyses of health-promoting bacteria: probiogenomics. Nat Rev Microbiol. 7: 61–71. [DOI] [PubMed] [Google Scholar]
- Wang Q, Garrity GM, Tiedje JM, and Cole JR (2007). Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitacre CC, Reingold SC, O’Looney PA, and Task Force on Gender, Multiple Sclerosis and Autoimmunity. (1999). A gender gap in autoimmunity. Science. 283: 1277–1278. [DOI] [PubMed] [Google Scholar]
- Wilcox MDP (2013). Characterization of the normal microbiota of the ocular surface. Exp Eye Res. 117: 99–105. [DOI] [PubMed] [Google Scholar]
- Wu GD, Lewis JD, Hoffmann C, Chen Y, Knight R, Bittinger K, et al. (2010). Sampling and pyrosequencing methods for characterizing bacterial communities in the human gut using 16S sequence tags. BMC Microbiology. 10: 206 Doi: 10.1186/1471-2180-10-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Fei Y, Qin Y, Luo D, Yang S, Kou X, et al. (2015). Bacterial flora changes in conjunctiva of rats with streptozotocin-induced type I diabetes mellitus. PLoS One. doi: 10.1371/journal.pone/0133021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, et al. (2013). Gender Bias in Autoimmunity Is Influenced by Microbiota. Immunity. 39: 400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandman-Goddard G, Peeva E, and Schoenfeld Y (2007). Gender and autoimmunity. Autoimmun Rev. 6: 366–372. [DOI] [PubMed] [Google Scholar]
- Zegans ME and Van Gelder RN (2014). Considerations in understanding the ocular surface microbiome. A J Ophthalmol. 158: 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R and Caspi RR (2010). Ocular immune privilege. Biol Rep. doi: 10.3410/B2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Holland MJ, Makalo P, Joof H, Roberts CH, Mabey DCW, et al. (2014). The conjunctival microbiome in health and trachomatous disease: a case control study. Genome Med. 6:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.